Distinction of papillary and adamantinomatous craniopharyngioma: Clinical features, surgical nuances and hypothalamic outcomes
- PMID: 39357265
- PMCID: PMC11474188
- DOI: 10.1016/j.neo.2024.101060
Distinction of papillary and adamantinomatous craniopharyngioma: Clinical features, surgical nuances and hypothalamic outcomes
Abstract
Objective: Understanding the differences of suprasellar papillary and adamantinomatous craniopharyngiomas (PCPs/ACPs) is pivotal for target therapy, surgical strategy or postoperative management. Here, the clinical features, surgical nuances and postoperative hypothalamic outcomes of PCPs were systematically recapitulated.
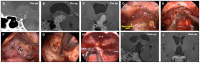
Methods: 24 PCPs and 52 ACPs underwent initial surgery were retrospectively reviewed. Clinical data, quantified third ventricle (3rd V) occupation and optic chiasm distortion were compared, as well as intra-operative findings, operating notes and prognosis. Moreover, analysis of tumor/3rd V relationship and hypothalamic outcomes were also performed.
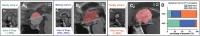
Results: Tumors were more likely to occupies the 3rd V cavity in PCPs. Chiasm distortion of "compressed forward" was the most common pattern (45.8 %) in PCPs, whereas "stretched forward" pattern accounted the highest (42.5 %) in ACPs. Besides, round-shaped with less calcification, duct-like recess, solid consistency, rare subdiaphragmatic invasion, visible lower stalk and improved postoperative visual outcome were more frequently observed in PCPs. The basal membranes of the tumor epithelium and the reactive gliosis were separated by a layer of collagen fibers in most PCPs, which differs from ACPs in the morphological examination of tumor/3rd V floor interface. In daytime sleepiness and memory difficulty, the PCPs showed significantly better outcomes than the ACPs groups, and PCPs suffered less postoperative weight gain (p < 0.05) than ACPs among adult-onset cases.
Conclusion: PCPs are different from ACPs regards the clinical features, operative techniques and outcomes. If necessary, PCPs are suggested more amenable to total removal since its less invasiveness to the 3rd V floor and better hypothalamic outcomes.
Keywords: Clinical characteristics; Hypothalamic outcomes; Papillary craniopharyngioma; Surgical technique; Third ventricle floor; Tumor topography.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

