Belantamab Mafodotin in Relapsed/Refractory AL Amyloidosis: Real-World Multi-Center Experience and Review of the Literature
- PMID: 39357511
- PMCID: PMC12306947
- DOI: 10.1159/000541594
Belantamab Mafodotin in Relapsed/Refractory AL Amyloidosis: Real-World Multi-Center Experience and Review of the Literature
Abstract
Introduction: Treatment for relapsed/refractory AL amyloidosis (AL) is an unmet need. The safety and efficacy of belantamab mafodotin (BLM) in multiple myeloma are known, whereas in AL data are limited.
Methods: We report a multi-center cohort of AL patients receiving BLM, and review all previous data on BLM therapy in AL.
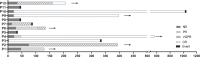
Results: Twelve patients with a median of 3 (range 2-9) prior lines of therapy were included. The overall hematological response rate (ORR) was 75% (9/12), including 5 complete responses. Six of the 10 evaluable patients had organ responses. The median event-free survivals/overall survivals were 22.3 and 28.8 months, respectively. Grade 3 toxicities were mostly infections and keratopathy, occurring in 7/12 (58%). Hematological toxicities were rare. No grade 4/5 toxicities occurred. The review of the previous series reveals BLM provides an ORR of 60-83% with similar rates of corneal toxicity.
Conclusion: BLM, being an off-the-shelf therapy, with acceptable toxicity even in frail patients, may be a valuable option in AL, with a high ORR, and a signal for durable responses and high-quality organ responses.
Keywords: AL amyloidosis; Antibody drug conjugate; BCMA; Belantamab mafodotin; Real-world experience.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Paolo Milani and Giovanni Palladini report participation in advisory boards for Alexion, Argobio, Janssen, and Protego; honoraria from Alexion, Argobio, Janssen, Protego, The Binding Site, Pfizer, Prothena, Sebia, and Siemens; and research funding from Gate Bioscience and The Binding Site. All the other authors declare no conflict of interest.
Figures
References
-
- Palladini G, Merlini G. How I treat AL amyloidosis. Blood. 2022;139(19):2918–30. - PubMed
-
- Gertz MA. Immunoglobulin light chain amyloidosis: 2022 update on diagnosis, prognosis, and treatment. Am J Hematol. 2022;97(6):818–29. - PubMed
-
- Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. - PubMed
-
- Mahmood S, Venner CP, Sachchithanantham S, Lane T, Rannigan L, Foard D, et al. Lenalidomide and dexamethasone for systemic AL amyloidosis following prior treatment with thalidomide or bortezomib regimens. Br J Haematol. 2014;166(6):842–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials