Extracellular vesicles bearing serum amyloid A1 exacerbate neuroinflammation after intracerebral haemorrhage
- PMID: 39357895
- PMCID: PMC12230225
- DOI: 10.1136/svn-2024-003525
Extracellular vesicles bearing serum amyloid A1 exacerbate neuroinflammation after intracerebral haemorrhage
Abstract
Introduction: Intracerebral haemorrhage (ICH) elicits a robust inflammatory response, which significantly contributes to secondary brain damage. Extracellular vesicles (EVs) play a pivotal role in intercellular communication by transporting immune-regulatory proteins. However, the precise contribution of these EV-carried proteins to neuroinflammation following ICH remains elusive. Here, we identified proteins dysregulated in EVs and further studied the EVs-enriched Serum amyloid A 1 (SAA1) to understand its role in neuroinflammation and ICH injury.
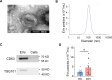
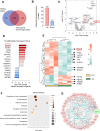
Methods: We used mass spectrometry to analyse the EV protein cargo isolated from plasma samples of 30 ICH patients and 30 healthy controls. To validate the function of the dysregulated protein SAA1, an ICH mouse model was conducted to assess the effects of SAA1 neutralisation on brain oedema, neurological function and infiltration of peripheral leucocytes.
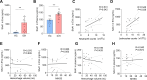
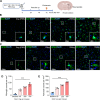
Results: 49 upregulated proteins and 12 downregulated proteins were observed in EVs from ICH patients compared with controls. Notably, SAA1 demonstrated a significant increase in EVs associated with ICH. We observed that exogenous SAA1 stimulation led to an augmentation in the population of microglia and astrocytes, exacerbating neuroinflammation. Neutralising SAA1 with an anti-SAA1 monoclonal antibody (mAb) diminished the prevalence of proinflammatory microglia and the infiltration of peripheral leucocytes, which ameliorates brain oedema and neurological function in ICH mice.
Conclusion: Our findings provide compelling evidence implicating EVs and their cargo proteins in ICH pathogenesis. SAA1 emerges as a potential therapeutic target for mitigating neuroinjury and neuroinflammation following ICH.
Keywords: Hemorrhage; Inflammation; Stroke.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60. doi: 10.1161/STR.0000000000000069. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
