Altered Protein Palmitoylation as Disease Mechanism in Neurodegenerative Disorders
- PMID: 39358031
- PMCID: PMC11450541
- DOI: 10.1523/JNEUROSCI.1225-24.2024
Altered Protein Palmitoylation as Disease Mechanism in Neurodegenerative Disorders
Abstract
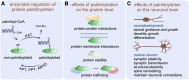
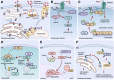
Palmitoylation, a lipid-based posttranslational protein modification, plays a crucial role in regulating various aspects of neuronal function through altering protein membrane-targeting, stabilities, and protein-protein interaction profiles. Disruption of palmitoylation has recently garnered attention as disease mechanism in neurodegeneration. Many proteins implicated in neurodegenerative diseases and associated neuronal dysfunction, including but not limited to amyloid precursor protein, β-secretase (BACE1), postsynaptic density protein 95, Fyn, synaptotagmin-11, mutant huntingtin, and mutant superoxide dismutase 1, undergo palmitoylation, and recent evidence suggests that altered palmitoylation contributes to the pathological characteristics of these proteins and associated disruption of cellular processes. In addition, dysfunction of enzymes that catalyze palmitoylation and depalmitoylation has been connected to the development of neurological disorders. This review highlights some of the latest advances in our understanding of palmitoylation regulation in neurodegenerative diseases and explores potential therapeutic implications.
Keywords: neurodegeneration; palmitoylation.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Lack of BACE1 S-palmitoylation reduces amyloid burden and mitigates memory deficits in transgenic mouse models of Alzheimer's disease.Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):E9665-E9674. doi: 10.1073/pnas.1708568114. Epub 2017 Oct 23. Proc Natl Acad Sci U S A. 2017. PMID: 29078331 Free PMC article.
-
Palmitoylation of synaptic proteins: roles in functional regulation and pathogenesis of neurodegenerative diseases.Cell Mol Biol Lett. 2024 Aug 10;29(1):108. doi: 10.1186/s11658-024-00625-2. Cell Mol Biol Lett. 2024. PMID: 39127627 Free PMC article. Review.
-
Protein S-Palmitoylation and Lung Diseases.Adv Exp Med Biol. 2021;1304:165-186. doi: 10.1007/978-3-030-68748-9_10. Adv Exp Med Biol. 2021. PMID: 34019269
-
S-palmitoylation of gamma-secretase subunits nicastrin and APH-1.J Biol Chem. 2009 Jan 16;284(3):1373-84. doi: 10.1074/jbc.M806380200. Epub 2008 Nov 20. J Biol Chem. 2009. PMID: 19028695 Free PMC article.
-
Palmitoylation in Alzheimer's disease and other neurodegenerative diseases.Pharmacol Res. 2016 Sep;111:133-151. doi: 10.1016/j.phrs.2016.06.008. Epub 2016 Jun 9. Pharmacol Res. 2016. PMID: 27293050 Review.
Cited by
-
In vitro reconstitution reveals substrate selectivity of protein S-acyltransferases.J Biol Chem. 2025 Apr;301(4):108406. doi: 10.1016/j.jbc.2025.108406. Epub 2025 Mar 14. J Biol Chem. 2025. PMID: 40090582 Free PMC article.
-
Reduced Palmitoylation of SQSTM1/p62 in Huntington Disease Is Associated With Impaired Autophagy.FASEB J. 2025 May 15;39(9):e70549. doi: 10.1096/fj.202401781R. FASEB J. 2025. PMID: 40326774 Free PMC article.
-
Palmitoylation-related gene ZDHHC22 as a potential diagnostic and immunomodulatory target in Alzheimer's disease: insights from machine learning analyses and WGCNA.Eur J Med Res. 2025 Jan 22;30(1):46. doi: 10.1186/s40001-025-02277-0. Eur J Med Res. 2025. PMID: 39844282 Free PMC article.
-
zDHHC-Mediated S-Palmitoylation in Skin Health and Its Targeting as a Treatment Perspective.Int J Mol Sci. 2025 Feb 15;26(4):1673. doi: 10.3390/ijms26041673. Int J Mol Sci. 2025. PMID: 40004137 Free PMC article. Review.
-
Stabilization of mitochondria-associated endoplasmic reticulum membranes regulates Aβ generation in a three-dimensional neural model of Alzheimer's disease.Alzheimers Dement. 2025 Feb;21(2):e14417. doi: 10.1002/alz.14417. Epub 2024 Dec 23. Alzheimers Dement. 2025. PMID: 39713841 Free PMC article.
References
-
- Abrar F, et al. (2023) Reduced S-acylation of SQSTM1/p62 in Huntington disease is associated with impaired autophagy. bioRxiv 561600.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
