Growth Hormone Receptor in Lateral Hypothalamic Neurons Is Required for Increased Food-Seeking Behavior during Food Restriction in Male Mice
- PMID: 39358046
- PMCID: PMC11580784
- DOI: 10.1523/JNEUROSCI.1761-23.2024
Growth Hormone Receptor in Lateral Hypothalamic Neurons Is Required for Increased Food-Seeking Behavior during Food Restriction in Male Mice
Abstract
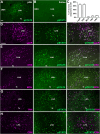
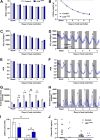
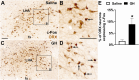
Growth hormone (GH) action in the brain regulates neuroendocrine axes, energy and glucose homeostasis, and several neurological functions. The lateral hypothalamic area (LHA) contains numerous neurons that respond to a systemic GH injection by expressing the phosphorylated STAT5, a GH receptor (GHR) signaling marker. However, the potential role of GHR signaling in the LHA is unknown. In this study, we demonstrated that ∼70% of orexin- and leptin receptor (LepR)-expressing neurons in the LHA are responsive to GH. Male mice carrying inactivation of the Ghr gene in the LHA were generated via bilateral injections of an adeno-associated virus. In ad libitum-fed mice, GHR ablation in LHA neurons did not significantly change energy and glucose homeostasis. Subsequently, mice were subjected to 5 d of 40% food restriction. Food restriction decreased body weight, energy expenditure, and carbohydrate oxidation. These effects were similarly observed in control and LHAΔGHR mice. While food-deprived control mice progressively increased ambulatory/exploratory activity and food-seeking behavior, LHAΔGHR mice did not show hyperactivity induced by food restriction. GHR ablation in the LHA reduced the percentage of orexin neurons expressing c-Fos during food restriction. Additionally, an acute GH injection increased the expression of c-Fos in LHAORX neurons. Inactivation of Ghr in LepR-expressing cells did not prevent hyperactivity in food-deprived mice, whereas whole-brain Ghr knock-out mice showed reduced ambulatory activity during food restriction. Our findings indicate that GHR signaling in the LHA regulates the activity of orexin neurons and is necessary to increase food-seeking behavior in food-deprived male mice.
Keywords: GH; arousal; food-seeking; hunger; hypocretin; orexin.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
