Cerebrospinal fluid flow in small-breed dogs with idiopathic epilepsy observed using time-spatial labeling inversion pulse images: a preliminary study
- PMID: 39358237
- PMCID: PMC11569881
- DOI: 10.1292/jvms.23-0305
Cerebrospinal fluid flow in small-breed dogs with idiopathic epilepsy observed using time-spatial labeling inversion pulse images: a preliminary study
Abstract
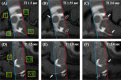
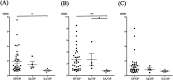
Cerebrospinal fluid (CSF) circulation diseases, such as hydrocephalus and syringomyelia, are common in small-breed dogs. In human patients with CSF circulation diseases, time-spatial labeling inversion pulse (time-SLIP) sequence performed to evaluate CSF flow before and after treatment allows visualization of the restoration of CSF movement. However, studies evaluating CSF flow using the time-SLIP method in small-breed dogs are limited. Therefore, the present study aimed to evaluate intracranial CSF flow on time-SLIP images in small-breed dogs with idiopathic epilepsy, as an alternative model to healthy dogs. Time-SLIP images were obtained at two sites: 1) the mesencephalic aqueduct (MA) area (third ventricle, MA, and brain-base subarachnoid space [SAS]) and 2) the craniocervical junction area (fourth ventricle, brainstem, and cervical spinal cord SAS) to allow subsequent evaluation of the rostral and caudal CSF flow using subjective and objective methods. In total, six dogs were included. Caudal flow at the MA and brain-base SAS and rostral flow in the brainstem SAS were subjectively and objectively observed in all and 5/6 dogs, respectively. Objective evaluation revealed that a significantly smaller movement of the CSF, assessed as the absence of CSF flow by subjective evaluation, could be detected in some areas. In small-breed dogs, the MA, brain-base, and brainstem SAS would be appropriate areas for evaluating CSF movement, either in the rostral or caudal flows on time-SLIP images. In areas where CSF movement cannot detected by subjective methods, an objective evaluation should be conducted.
Keywords: canine; cerebrospinal fluid; magnetic resonance imaging; syringomyelia; time-spatial labeling inversion pulse.
Conflict of interest statement
None of the authors have any conflicts of interest to declare.
Figures


References
-
- Bessen M, Gayen C, Quarrington R, Walls A, Doig R, Dorrian R, Leonard A, Kurtcuoglu V, Jones CF. 2023. 96. Spinal cerebrospinal fluid flow and intrathecal occlusion following traumatic spinal cord injury in a porcine model. Spine J 23: S49–S50. doi: 10.1016/j.spinee.2023.06.149 - DOI

