Aquatic environment drives the emergence of cell wall-deficient dormant forms in Listeria
- PMID: 39358320
- PMCID: PMC11447242
- DOI: 10.1038/s41467-024-52633-7
Aquatic environment drives the emergence of cell wall-deficient dormant forms in Listeria
Abstract
Stressed bacteria can enter a dormant viable but non-culturable (VBNC) state. VBNC pathogens pose an increased health risk as they are undetectable by growth-based techniques and can wake up back into a virulent state. Although widespread in bacteria, the mechanisms governing this phenotypic switch remain elusive. Here, we investigate the VBNC state transition in the human pathogen Listeria monocytogenes. We show that bacteria starved in mineral water become VBNC by converting into osmotically stable cell wall-deficient coccoid forms, a phenomenon that occurs in other Listeria species. We reveal the bacterial stress response regulator SigB and the autolysin NamA as major actors of VBNC state transition. We lastly show that VBNC Listeria revert to a walled and virulent state after passage in chicken embryos. Our study provides more detail on the VBNC state transition mechanisms, revealing wall-free bacteria naturally arising in aquatic environments as a potential survival strategy in hypoosmotic and oligotrophic conditions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

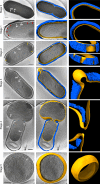


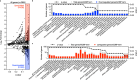
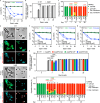
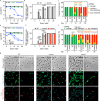

References
-
- Dong, K. et al. Induction, detection, formation, and resuscitation of viable but non-culturable state microorganisms. Compr. Rev. food Sci. food Saf.19, 149–183 (2020). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

