Intrinsic temperature increase drives lipid metabolism towards ferroptosis evasion and chemotherapy resistance in pancreatic cancer
- PMID: 39358362
- PMCID: PMC11447004
- DOI: 10.1038/s41467-024-52978-z
Intrinsic temperature increase drives lipid metabolism towards ferroptosis evasion and chemotherapy resistance in pancreatic cancer
Abstract
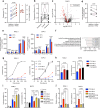
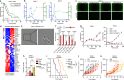
A spontaneously occurring temperature increase in solid tumors has been reported sporadically, but is largely overlooked in terms of cancer biology. Here we show that temperature is increased in tumors of patients with pancreatic ductal adenocarcinoma (PDAC) and explore how this could affect therapy response. By mimicking this observation in PDAC cell lines, we demonstrate that through adaptive changes in lipid metabolism, the temperature increase found in human PDAC confers protection to lipid peroxidation and contributes to gemcitabine resistance. Consistent with the recently uncovered role of p38 MAPK in ferroptotic cell death, we find that the reduction in lipid peroxidation potential following adaptation to tumoral temperature allows for p38 MAPK inhibition, conferring chemoresistance. As an increase in tumoral temperature is observed in several other tumor types, our findings warrant taking tumoral temperature into account in subsequent studies related to ferroptosis and therapy resistance. More broadly, our findings indicate that tumoral temperature affects cancer biology.
© 2024. The Author(s).
Conflict of interest statement
T.V.B. and K.A. hold patents US9862678, WO2016075330, EP3218357, and WO2019154795 related to ferrostatin-1 analogs. The remaining authors declare no competing interests.
Figures


References
-
- Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell144, 646–674 (2011). - PubMed
-
- Gautherie, M. Thermopathology of breast cancer: measurement and analysis of in vivo temperature and blood flow. Ann. N. Y. Acad. Sci.335, 383–415 (1980). - PubMed
-
- Bousselham, A., Bouattane, O., Youssfi, M. & Raihani, A. Brain tumor temperature effect extraction from MRI imaging using bioheat equation. Proc. Comput. Sci.127, 336–343 (2018).
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

