Minimal exposure durations reveal visual processing priorities for different stimulus attributes
- PMID: 39358365
- PMCID: PMC11447214
- DOI: 10.1038/s41467-024-52778-5
Minimal exposure durations reveal visual processing priorities for different stimulus attributes
Abstract
Human vision can detect a single photon, but the minimal exposure required to extract meaning from stimulation remains unknown. This requirement cannot be characterised by stimulus energy, because the system is differentially sensitive to attributes defined by configuration rather than physical amplitude. Determining minimal exposure durations required for processing various stimulus attributes can thus reveal the system's priorities. Using a tachistoscope enabling arbitrarily brief displays, we establish minimal durations for processing human faces, a stimulus category whose perception is associated with several well-characterised behavioural and neural markers. Neural and psychophysical measures show a sequence of distinct minimal exposures for stimulation detection, object-level detection, face-specific processing, and emotion-specific processing. Resolving ongoing debates, face orientation affects minimal exposure but emotional expression does not. Awareness emerges with detection, showing no evidence of subliminal perception. These findings inform theories of visual processing and awareness, elucidating the information to which the visual system is attuned.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


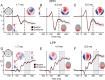
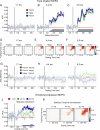


References
-
- Hecht, S., Shlaer, S. & Pirenne, M. H. Energy at the threshold of vision. Science93, 585–587 (1941). - PubMed
-
- Fabre-Thorpe, M., Delorme, A., Marlot, C. & Thorpe, S. A limit to the speed of processing in ultra-rapid visual categorization of novel natural scenes. J. Cogn. Neurosci.13, 171–180 (2001). - PubMed
-
- Thorpe, S., Fize, D. & Marlot, C. Speed of processing in the human visual system. Nature381, 520–522 (1996). - PubMed
Publication types
MeSH terms
Grants and funding
- 1240899/Ministry of Education, Government of Chile | Comisión Nacional de Investigación Científica y Tecnológica (National Commission for Scientific and Technological Research)
- ES/L01064X/1/RCUK | Economic and Social Research Council (ESRC)
- EXPERIENCE - ADG101055060/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- EXPERIENCE - ADG101055060/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources

