Grain boundary engineering for efficient and durable electrocatalysis
- PMID: 39358376
- PMCID: PMC11446910
- DOI: 10.1038/s41467-024-52919-w
Grain boundary engineering for efficient and durable electrocatalysis
Abstract
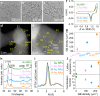
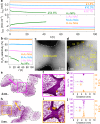
Grain boundaries in noble metal catalysts have been identified as critical sites for enhancing catalytic activity in electrochemical reactions such as the oxygen reduction reaction. However, conventional methods to modify grain boundary density often alter particle size, shape, and morphology, obscuring the specific role of grain boundaries in catalytic performance. This study addresses these challenges by employing gold nanoparticle assemblies to control grain boundary density through the manipulation of nanoparticle collision frequency during synthesis. We demonstrate a direct correlation between increased grain boundary density and enhanced two-electron oxygen reduction reaction activity, achieving a significant improvement in both specific and mass activity. Additionally, the gold nanoparticle assemblies with high grain boundary density exhibit remarkable electrochemical stability, attributed to boron segregation at the grain boundaries, which prevents structural degradation. This work provides a promising strategy for optimizing the activity, selectivity, and stability of noble metal catalysts through precise grain boundary engineering.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Kibsgaard, J. & Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy4, 430–433 (2019).
-
- He, T. et al. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature598, 76–81 (2021). - PubMed
-
- Huang, W. et al. Steam-created grain boundaries for methane C–H activation in palladium catalysts. Science373, 1518–1523 (2021). - PubMed
-
- Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature508, 504–507 (2014). - PubMed
-
- Li, Z. et al. A silver catalyst activated by stacking faults for the hydrogen evolution reaction. Nat. Catal.2, 1107–1114 (2019).

