Phase III randomized clinical trial of efficacy and safety of amlodipine and candesartan cilexetil combination for hypertension treatment
- PMID: 39358448
- PMCID: PMC11447083
- DOI: 10.1038/s41598-024-74003-5
Phase III randomized clinical trial of efficacy and safety of amlodipine and candesartan cilexetil combination for hypertension treatment
Abstract
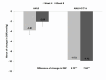
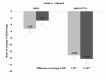
Effective antihypertensive therapy is essential for achieving optimal blood pressure (BP) control and reducing cardiovascular events. This double-blind, multicenter, randomized trial aimed to compare the antihypertensive efficacy and safety of a combination of amlodipine (AML) and candesartan cilexetil (CC) versus AML monotherapy in patients with essential hypertension (HTN). After a 4-week run-in period with AML 5 mg, patients whose HTN remained uncontrolled (diastolic BP [DBP]) ≥ 90 mmHg and < 120 mmHg) were randomized to receive either AML + CC or AML alone for 8 weeks. Efficacy was assessed by measuring changes in DBP and systolic BP (SBP). The primary safety measure was the incidence of adverse events (AEs). A total of 174 participants were included in the efficacy analysis. After 8 weeks, DBP decreased by -9.92 ± 0.86 mmHg in the AML + CC arm and - 2.08 ± 0.86 mmHg in the AML arm (p < 0.0001). SBP decreased by -14.27 ± 1.39 mmHg in the AML + CC arm versus - 2.77 ± 1.39 mmHg in the AML arm (p < 0.0001). AEs occurred in 11.24% of the AML + CC group and 5.62% of the AML group (p = 0.1773). AML + CC combination therapy demonstrated superior efficacy with good tolerance, making it a promising option for patients with inadequately controlled hypertension on amlodipine alone.
Keywords: Amlodipine; Angiotensin receptor blocker; Antihypertensive; Blood pressure; Candesartan Cilexetil; HTN.
© 2024. The Author(s).
Conflict of interest statement
This study was funded by HK inno.N Corp. (formerly CJ Healthcare), Seoul, Korea. The sponsor was responsible for data collation and statistical analysis, which was supported by Chung Hyun Choi, an employee of HK inno.N Corp. Soohong Kim, an employee of HK inno.N Corp., assisted with the preparation of the manuscript, but did not meet the criteria for authorship. The authors declare no other competing interests.
Figures


References
-
- Mancia, G. et al. 2023 ESH guidelines for the management of arterial hypertension the Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens.41, 1874–2071 (2023). - DOI - PubMed
-
- Whelton, P. K. et al. ACC/AHA/AAPA/ABC/ACPM/AGS. A/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 324, ;71:1269 (2017) APh. (2018). - PubMed
-
- Unger, T. et al. International Society of Hypertension global hypertension practice guidelines. J. Hypertens. 38, 982–1004 (2020). (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

