Recording morphogen signals reveals mechanisms underlying gastruloid symmetry breaking
- PMID: 39358450
- PMCID: PMC11806519
- DOI: 10.1038/s41556-024-01521-9
Recording morphogen signals reveals mechanisms underlying gastruloid symmetry breaking
Abstract
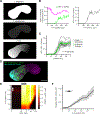
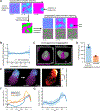
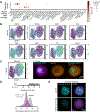
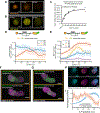
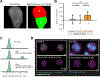
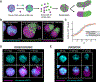
Aggregates of stem cells can break symmetry and self-organize into embryo-like structures with complex morphologies and gene expression patterns. Mechanisms including reaction-diffusion Turing patterns and cell sorting have been proposed to explain symmetry breaking but distinguishing between these candidate mechanisms of self-organization requires identifying which early asymmetries evolve into subsequent tissue patterns and cell fates. Here we use synthetic 'signal-recording' gene circuits to trace the evolution of signalling patterns in gastruloids, three-dimensional stem cell aggregates that form an anterior-posterior axis and structures resembling the mammalian primitive streak and tailbud. We find that cell sorting rearranges patchy domains of Wnt activity into a single pole that defines the gastruloid anterior-posterior axis. We also trace the emergence of Wnt domains to earlier heterogeneity in Nodal activity even before Wnt activity is detectable. Our study defines a mechanism through which aggregates of stem cells can form a patterning axis even in the absence of external spatial cues.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures











Update of
-
Recording morphogen signals reveals origins of gastruloid symmetry breaking.bioRxiv [Preprint]. 2023 Jun 5:2023.06.02.543474. doi: 10.1101/2023.06.02.543474. bioRxiv. 2023. Update in: Nat Cell Biol. 2024 Nov;26(11):1832-1844. doi: 10.1038/s41556-024-01521-9. PMID: 37333235 Free PMC article. Updated. Preprint.
References
-
- Beccari L et al. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276 (2018). - PubMed
Methods-only References
-
- Thielicke W & Sonntag R Particle Image Velocimetry for MATLAB: Accuracy and enhanced algorithms in PIVlab. Journal of Open Research Software 9, (2021).
MeSH terms
Substances
Grants and funding
- T32GM007388/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- U01 DK127429/DK/NIDDK NIH HHS/United States
- F31 HD113443/HD/NICHD NIH HHS/United States
- 2134935/NSF | Directorate for Biological Sciences (BIO)
- 1734030/NSF | Directorate for Mathematical & Physical Sciences | Division of Physics (PHY)
LinkOut - more resources
Full Text Sources
Research Materials

