Calcium-permeable AMPA receptors govern PV neuron feature selectivity
- PMID: 39358515
- PMCID: PMC11560848
- DOI: 10.1038/s41586-024-08027-2
Calcium-permeable AMPA receptors govern PV neuron feature selectivity
Abstract
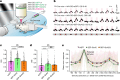
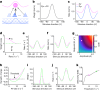
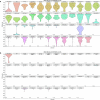
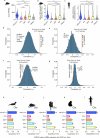
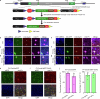
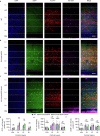
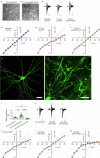
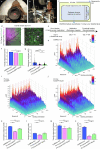
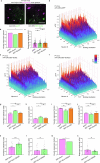
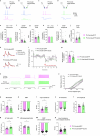
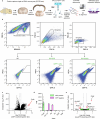
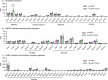
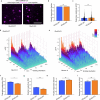
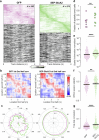
The brain helps us survive by forming internal representations of the external world1,2. Excitatory cortical neurons are often precisely tuned to specific external stimuli3,4. However, inhibitory neurons, such as parvalbumin-positive (PV) interneurons, are generally less selective5. PV interneurons differ from excitatory neurons in their neurotransmitter receptor subtypes, including AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors (AMPARs)6,7. Excitatory neurons express calcium-impermeable AMPARs that contain the GluA2 subunit (encoded by GRIA2), whereas PV interneurons express receptors that lack the GluA2 subunit and are calcium-permeable (CP-AMPARs). Here we demonstrate a causal relationship between CP-AMPAR expression and the low feature selectivity of PV interneurons. We find low expression stoichiometry of GRIA2 mRNA relative to other subunits in PV interneurons that is conserved across ferrets, rodents, marmosets and humans, and causes abundant CP-AMPAR expression. Replacing CP-AMPARs in PV interneurons with calcium-impermeable AMPARs increased their orientation selectivity in the visual cortex. Manipulations to induce sparse CP-AMPAR expression demonstrated that this increase was cell-autonomous and could occur with changes beyond development. Notably, excitatory-PV interneuron connectivity rates and unitary synaptic strength were unaltered by CP-AMPAR removal, which suggested that the selectivity of PV interneurons can be altered without markedly changing connectivity. In Gria2-knockout mice, in which all AMPARs are calcium-permeable, excitatory neurons showed significantly degraded orientation selectivity, which suggested that CP-AMPARs are sufficient to drive lower selectivity regardless of cell type. Moreover, hippocampal PV interneurons, which usually exhibit low spatial tuning, became more spatially selective after removing CP-AMPARs, which indicated that CP-AMPARs suppress the feature selectivity of PV interneurons independent of modality. These results reveal a new role of CP-AMPARs in maintaining low-selectivity sensory representation in PV interneurons and implicate a conserved molecular mechanism that distinguishes this cell type in the neocortex.
© 2024. The Author(s).
Conflict of interest statement
Figures








References
-
- Olshausen, B. A. & Field, D. J. Sparse coding of sensory inputs. Curr. Opin. Neurobiol.14, 481–487 (2004). - PubMed
-
- Destexhe, A. & Marder, E. Plasticity in single neuron and circuit computations. Nature431, 789–795 (2004). - PubMed
-
- Niell, C. M. Cell types, circuits, and receptive fields in the mouse visual cortex. Annu. Rev. Neurosci.38, 413–431 (2015). - PubMed
-
- Hu, H., Gan, J. & Jonas, P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science345, 1255263 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

