Neuronal parts list and wiring diagram for a visual system
- PMID: 39358525
- PMCID: PMC11446827
- DOI: 10.1038/s41586-024-07981-1
Neuronal parts list and wiring diagram for a visual system
Abstract
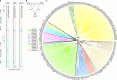
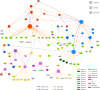
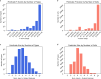
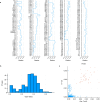
A catalogue of neuronal cell types has often been called a 'parts list' of the brain1, and regarded as a prerequisite for understanding brain function2,3. In the optic lobe of Drosophila, rules of connectivity between cell types have already proven to be essential for understanding fly vision4,5. Here we analyse the fly connectome to complete the list of cell types intrinsic to the optic lobe, as well as the rules governing their connectivity. Most new cell types contain 10 to 100 cells, and integrate information over medium distances in the visual field. Some existing type families (Tm, Li, and LPi)6-10 at least double in number of types. A new serpentine medulla (Sm) interneuron family contains more types than any other. Three families of cross-neuropil types are revealed. The consistency of types is demonstrated by analysing the distances in high-dimensional feature space, and is further validated by algorithms that select small subsets of discriminative features. We use connectivity to hypothesize about the functional roles of cell types in motion, object and colour vision. Connectivity with 'boundary types' that straddle the optic lobe and central brain is also quantified. We showcase the advantages of connectomic cell typing: complete and unbiased sampling, a rich array of features based on connectivity and reduction of the connectome to a substantially simpler wiring diagram of cell types, with immediate relevance for brain function and development.
© 2024. The Author(s).
Conflict of interest statement
H.S.S. declares financial interests in Zetta AI. The other authors declare no competing interests.
Figures











Update of
-
Neuronal "parts list" and wiring diagram for a visual system.bioRxiv [Preprint]. 2024 Apr 15:2023.10.12.562119. doi: 10.1101/2023.10.12.562119. bioRxiv. 2024. Update in: Nature. 2024 Oct;634(8032):166-180. doi: 10.1038/s41586-024-07981-1. PMID: 37873160 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

