Network statistics of the whole-brain connectome of Drosophila
- PMID: 39358527
- PMCID: PMC11446825
- DOI: 10.1038/s41586-024-07968-y
Network statistics of the whole-brain connectome of Drosophila
Abstract
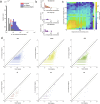
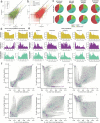
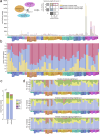
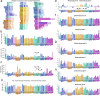
Brains comprise complex networks of neurons and connections, similar to the nodes and edges of artificial networks. Network analysis applied to the wiring diagrams of brains can offer insights into how they support computations and regulate the flow of information underlying perception and behaviour. The completion of the first whole-brain connectome of an adult fly, containing over 130,000 neurons and millions of synaptic connections1-3, offers an opportunity to analyse the statistical properties and topological features of a complete brain. Here we computed the prevalence of two- and three-node motifs, examined their strengths, related this information to both neurotransmitter composition and cell type annotations4,5, and compared these metrics with wiring diagrams of other animals. We found that the network of the fly brain displays rich-club organization, with a large population (30% of the connectome) of highly connected neurons. We identified subsets of rich-club neurons that may serve as integrators or broadcasters of signals. Finally, we examined subnetworks based on 78 anatomically defined brain regions or neuropils. These data products are shared within the FlyWire Codex ( https://codex.flywire.ai ) and should serve as a foundation for models and experiments exploring the relationship between neural activity and anatomical structure.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










Update of
-
Network Statistics of the Whole-Brain Connectome of Drosophila.bioRxiv [Preprint]. 2024 Feb 28:2023.07.29.551086. doi: 10.1101/2023.07.29.551086. bioRxiv. 2024. Update in: Nature. 2024 Oct;634(8032):153-165. doi: 10.1038/s41586-024-07968-y. PMID: 37547019 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

