The relevance of tumor target expression levels on IgA-mediated cytotoxicity in cancer immunotherapy
- PMID: 39358557
- PMCID: PMC11447191
- DOI: 10.1007/s00262-024-03824-0
The relevance of tumor target expression levels on IgA-mediated cytotoxicity in cancer immunotherapy
Abstract
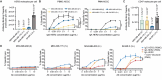
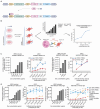
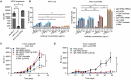
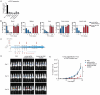
Recent advances in cancer immunotherapy, particularly the success of immune checkpoint inhibitors, have reignited interest in targeted monoclonal antibodies for immunotherapy. Antibody therapies aim to minimize on-target, off-tumor toxicity by targeting antigens overexpressed on tumor cells but not on healthy cells. Despite considerable efforts, some therapeutic antibodies have been linked to dose-limiting side effects. Our hypothesis suggests that the efficacy of IgG leads to a lower target expression threshold for tumor cell killing, contributing to these side effects. Earlier, therapeutic IgG antibodies were reformatted into the IgA isotype. Unlike IgG, which primarily engages Fc gamma receptors (FcγR) to induce antibody-dependent cellular cytotoxicity (ADCC) by NK cells and antibody-dependent cellular phagocytosis (ADCP) by monocytes/macrophages, IgA antibodies activate neutrophils through the Fc alpha receptor I (CD89, FcαRI). In previous studies, it appeared that IgA may require a higher target expression threshold for effective killing, and we aimed to investigate this in our current study. Moreover, we investigated how blocking the myeloid checkpoint CD47/SIRPα axis affect the target expression threshold. Using a tetracycline-inducible expression system, we regulated target expression in different cell lines. Our findings from ADCC assays indicate that IgA-mediated PMN ADCC requires a higher antigen expression level than IgG-mediated PBMC ADCC. Furthermore, blocking CD47 enhanced IgA-mediated ADCC, lowering the antigen threshold. Validated in two in vivo models, our results show that IgA significantly reduces tumor growth in high-antigen-expressing tumors without affecting low-antigen-expressing healthy tissues. This suggests IgA-based immunotherapy could potentially minimize on-target, off-tumor side effects, improving treatment efficacy and patient safety.
Keywords: Antigen expression levels; CD47/SIRPα; IgA; Immunotherapy; Neutrophils.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcαRI (CD89) binding.MAbs. 2015;7(4):743-51. doi: 10.1080/19420862.2015.1047570. MAbs. 2015. PMID: 25970007 Free PMC article.
-
Potent Fc Receptor Signaling by IgA Leads to Superior Killing of Cancer Cells by Neutrophils Compared to IgG.Front Immunol. 2019 Apr 11;10:704. doi: 10.3389/fimmu.2019.00704. eCollection 2019. Front Immunol. 2019. PMID: 31031746 Free PMC article.
-
IgA-Mediated Killing of Tumor Cells by Neutrophils Is Enhanced by CD47-SIRPα Checkpoint Inhibition.Cancer Immunol Res. 2020 Jan;8(1):120-130. doi: 10.1158/2326-6066.CIR-19-0144. Epub 2019 Nov 5. Cancer Immunol Res. 2020. PMID: 31690649
-
Macrophages are critical effectors of antibody therapies for cancer.MAbs. 2015;7(2):303-10. doi: 10.1080/19420862.2015.1011450. MAbs. 2015. PMID: 25667985 Free PMC article. Review.
-
Cancer immunotherapy targeting the CD47/SIRPα axis.Eur J Cancer. 2017 May;76:100-109. doi: 10.1016/j.ejca.2017.02.013. Epub 2017 Mar 10. Eur J Cancer. 2017. PMID: 28286286 Review.
Cited by
-
The Efficacy of Targeted Monoclonal IgA Antibodies Against Pancreatic Ductal Adenocarcinoma.Cells. 2025 Apr 24;14(9):632. doi: 10.3390/cells14090632. Cells. 2025. PMID: 40358156 Free PMC article.
References
-
- Zinn S, Vazquez-Lombardi R, Zimmermann C, Sapra P, Jermutus L, Christ D (2023) Advances in antibody-based therapy in oncology. Nat Cancer 4:165–180 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

