Elucidating siRNA Cellular Delivery Mechanism Mediated by Quaternized Starch Nanoparticles
- PMID: 39359045
- PMCID: PMC11657042
- DOI: 10.1002/smll.202405524
Elucidating siRNA Cellular Delivery Mechanism Mediated by Quaternized Starch Nanoparticles
Abstract
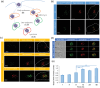
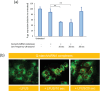
Starch-based nanoparticles are highly utilized in the realm of drug delivery taking advantage of their biocompatibility and biodegradability. Studies have utilized Quaternized starch (Q-starch) for small interfering RNA (siRNA) delivery, in which quaternary amines enable interaction with negatively charged siRNA, resulting in self-assembly complexation. Although reports present numerous applications, the demonstrated efficacy is nonetheless limited due to undiscovered cellular mechanistic delivery. In this study, a deep dive into Q-starch/siRNA complexes' cellular mechanism and kinetics at the cellular level is revealed using single-particle tracking and cell population level using imaging flow cytometry. Uptake studies depict the efficient cellular internalization via endocytosis while a significant fraction of complexes' intracellular fate is lysosome. Utilizing single-particle tracking, it is found that an average of 15% of cellular detected complexes escape the endosome which holds the potential for the integration in the cytoplasmatic gene silencing mechanism. Additional experimental manipulations (overcoming endosomal escape) demonstrate that the complex's disassembly is the rate-limiting step, correlating Q-starch's structure-function properties as siRNA carrier. Structure-function properties accentuating the high affinity of the interaction between Q-starch's quaternary groups and siRNA's phosphate groups that results in low release efficiency. However, low-frequency ultrasound (20 kHz) application may have induced siRNA release resulting in faster gene silencing kinetics.
Keywords: delivery mechanism; endocytosis; particle tracking; polysaccharide; siRNA; starch.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

