Discovery of a polyketide carboxylate phytotoxin from a polyketide glycoside hybrid by β-glucosidase mediated ester bond hydrolysis
- PMID: 39360009
- PMCID: PMC11441467
- DOI: 10.1039/d4sc05256k
Discovery of a polyketide carboxylate phytotoxin from a polyketide glycoside hybrid by β-glucosidase mediated ester bond hydrolysis
Abstract
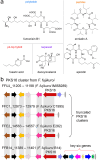
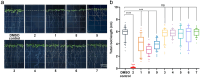
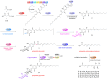
Fungal phytotoxins cause significant harm to agricultural production or lead to plant diseases. Discovering new phytotoxins, dissecting their formation mechanism and understanding their action mode are important for controlling the harmful effects of fungal phytopathogens. In this study, a long-term unsolved cluster (polyketide synthase 16, PKS16 cluster) from Fusarium species was thoroughly investigated and a series of new metabolites including both complex α-pyrone-polyketide glycosides and simple polyketide carboxylates were identified from F. proliferatum. The whole pathway reveals an unusual assembly and inactivation process for phytotoxin biosynthesis, with key points as follows: (1) a flavin dependent monooxygenase catalyzes Baeyer-Villiger oxidation on the linear polyketide side chain of α-pyrone-polyketide glycoside 8 to form ester bond compound 1; (2) a β-glucosidase unexpectedly mediates the ester bond hydrolysis of 1 to generate polyketide carboxylate phytotoxin 2; (3) oxidation occurring on the terminal inert carbons of 2 by intracellular oxidase(s) eliminates its phytotoxicity. Our work identifies the chemical basis of the PKS16 cluster in phytotoxicity, shows that polyketide carboxylate is a new structural type of phytotoxin in Fusarium and importantly uncovers a rare ester bond hydrolysis function of β-glucosidase family enzymes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Hohn T. M., in Plant Relationships: Part A, ed. G. C. Carroll and P. Tudzynski, Springer Berlin Heidelberg, Berlin, Heidelberg, 1997, pp. 129–144
-
- Huang J.-S., in Plant Pathogenesis and Resistance: Biochemistry and Physiology of Plant-Microbe Interactions, ed. J.-S. Huang, Springer Netherlands, Dordrecht, 2001, pp. 291–411
LinkOut - more resources
Full Text Sources

