Two-dimensional nanomaterials based on rare earth elements for biomedical applications
- PMID: 39360014
- PMCID: PMC11441461
- DOI: 10.1039/d4sc02625j
Two-dimensional nanomaterials based on rare earth elements for biomedical applications
Abstract
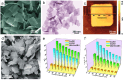
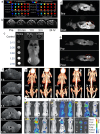
As a kind of star materials, two-dimensional (2D) nanomaterials have attracted tremendous attention for their unique structures, excellent performance and wide applications. In recent years, layered rare earth-based or doped nanomaterials have become a new important member of the 2D nanomaterial family and have attracted significant interest, especially layered rare earth hydroxides (LREHs) and layered rare earth-doped perovskites with anion-exchangeability and exfoliative properties. In this review, we systematically summarize the synthesis, exfoliation, fabrication and biomedical applications of 2D rare earth nanomaterials. Upon exfoliation, the LREHs and layered rare earth-doped perovskites can be dimensionally reduced to ultrathin nanosheets which feature high anisotropy and flexibility. Subsequent fabrication, especially superlattice assembly, enables rare earth nanomaterials with diverse compositions and structures, which further optimizes or even creates new properties and thus expands the application fields. The latest progress in biomedical applications of the 2D rare earth-based or doped nanomaterials and composites is also reviewed in detail, especially drug delivery and magnetic resonance imaging (MRI). Moreover, at the end of this review, we provide an outlook on the opportunities and challenges of the 2D rare earth-based or doped nanomaterials. We believe this review will promote increasing interest in 2D rare earth materials and provide more insight into the artificial design of other nanomaterials based on rare earth elements for functional applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures












Similar articles
-
Ultrathin 2D Rare-Earth Nanomaterials: Compositions, Syntheses, and Applications.Adv Mater. 2020 Jan;32(3):e1806461. doi: 10.1002/adma.201806461. Epub 2019 Apr 24. Adv Mater. 2020. PMID: 31018020 Review.
-
Two-dimensional nanomaterials: fascinating materials in biomedical field.Sci Bull (Beijing). 2019 Nov 30;64(22):1707-1727. doi: 10.1016/j.scib.2019.09.021. Epub 2019 Sep 20. Sci Bull (Beijing). 2019. PMID: 36659785 Review.
-
Rare-earth-containing perovskite nanomaterials: design, synthesis, properties and applications.Chem Soc Rev. 2020 Feb 24;49(4):1109-1143. doi: 10.1039/c9cs00330d. Chem Soc Rev. 2020. PMID: 31939973 Review.
-
Two-Dimensional Nanomaterial-Templated Composites.Acc Chem Res. 2022 Dec 20;55(24):3581-3593. doi: 10.1021/acs.accounts.2c00579. Epub 2022 Dec 7. Acc Chem Res. 2022. PMID: 36475610
-
Layered rare earth hydroxides (LREHs): synthesis and structure characterization towards multifunctionality.Dalton Trans. 2014 Jul 21;43(27):10355-64. doi: 10.1039/c4dt00425f. Epub 2014 May 14. Dalton Trans. 2014. PMID: 24824303
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

