4,5-Diamino-2-Thiouracil-Powered Dual-Mode Biosensor for Sensitive, Nonenzymatic Determination of Saliva Uric Acid Levels
- PMID: 39360016
- PMCID: PMC11446619
- DOI: 10.1155/2024/9944426
4,5-Diamino-2-Thiouracil-Powered Dual-Mode Biosensor for Sensitive, Nonenzymatic Determination of Saliva Uric Acid Levels
Abstract
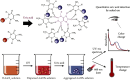
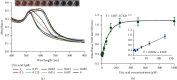
Nonenzymatic and rapid monitoring of uric acid levels is of great value for early diagnosis, prevention, and management of oxidative stress-associated diseases. However, fast, convenient, and low-cost uric acid detection remains challenging, especially in resource-limited settings. In this study, a novel and rapid biosensing approach was developed for the simultaneous visualization and quantification of uric acid levels by using the unique surface plasmon resonance and photothermal property of 4,5-diamino-2-thiouracil (DT)-capped gold nanoparticles (AuNPs). With the presence of uric acid, DT-capped AuNPs rapidly aggregated, and a visible color/photothermal change was used for uric acid quantification within 15 min. The limit of detection was determined to be 11.3 and 6.6 μM for the dual-mode biosensor, leveraging the unique structure of DT to optimize reaction kinetics. Moreover, the sensor exhibited excellent anti-interference capabilities and demonstrated potential for detecting a wide range of uric acid concentrations in complex samples, thereby reducing the need for extensive sample dilution and complex material synthesis procedures. Furthermore, validation against gold standard testing indicates that this biosensor could serve as a highly sensitive assay for quantifying uric acid levels in point-of-care applications, particularly in resource-limited settings.
Keywords: 4,5-diamino-2-thiouracil; colorimetric; nonenzymatic; photothermal; uric acid.
Copyright © 2024 Zipeng Wu et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
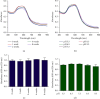
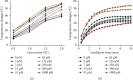
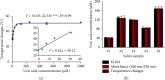
Similar articles
-
A luminescent MOF-based nonenzymatic probe for colorimetric/photothermal/fluorescence triple-mode assay of uric acid in body fluids.Talanta. 2024 Jan 15;267:125201. doi: 10.1016/j.talanta.2023.125201. Epub 2023 Sep 15. Talanta. 2024. PMID: 37722345
-
Enzyme-free colorimetric assay of serum uric acid.Chem Commun (Camb). 2011 Nov 7;47(41):11498-500. doi: 10.1039/c1cc13349g. Epub 2011 Sep 20. Chem Commun (Camb). 2011. PMID: 21935519
-
Disposable Nonenzymatic Uric Acid and Creatinine Sensors Using μPAD Coupled with Screen-Printed Reduced Graphene Oxide-Gold Nanocomposites.Int J Anal Chem. 2019 Feb 3;2019:3457247. doi: 10.1155/2019/3457247. eCollection 2019. Int J Anal Chem. 2019. PMID: 30853986 Free PMC article.
-
Colorimetric and photothermal immunosensor for sensitive detection of cancer biomarkers based on enzyme-mediated growth of gold nanostars on polydopamine.Anal Chim Acta. 2023 Oct 23;1279:341775. doi: 10.1016/j.aca.2023.341775. Epub 2023 Sep 1. Anal Chim Acta. 2023. PMID: 37827632
-
Perspectives of characterization and bioconjugation of gold nanoparticles and their application in lateral flow immunosensing.Drug Deliv Transl Res. 2020 Aug;10(4):878-902. doi: 10.1007/s13346-020-00771-y. Drug Deliv Transl Res. 2020. PMID: 32367423 Review.
References
-
- Vernerová A., Kujovská Krmová L., Melichar B., Vec F. Non-Invasive Determination of Uric Acid in Human Saliva in the Diagnosis of Serious Disorders. Clinical Chemistry and Laboratory Medicine . 2021;59:797–812. - PubMed
LinkOut - more resources
Full Text Sources

