Stage III NSCLC treatment options: too many choices
- PMID: 39360027
- PMCID: PMC11444491
- DOI: 10.1183/20734735.0047-2024
Stage III NSCLC treatment options: too many choices
Abstract
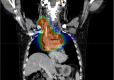
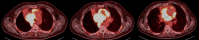
Stage III nonsmall cell lung cancer (NSCLC) represents a wide range of tumour (T1 to T4) and nodal (N0 to N3) components, requiring variable management and a multidisciplinary approach. Recent advancements in minimally invasive techniques, molecular biology and novel drug discoveries have accelerated the refinement of stage III NSCLC management. The latest developments in staging include the forthcoming update of the nodal component in the 9th TNM (tumour-node-metastasis) edition, which emphasises the critical role for endobronchial ultrasonography in mediastinal staging. Recent treatment developments include the use of immunotherapy and targeted molecular therapy in both the neoadjuvant and adjuvant setting, either in combination with other modalities or used alone as consolidation. Surgical and radiotherapy advancements have further enhanced patient outcomes. These developments have significantly improved the prognosis for patients with stage III NSCLC. Fast-changing recommendations have also brought about a challenge, with clinicians facing a number of options to choose from. Therefore, a multimodal approach by a multidisciplinary team has become even more crucial in managing stage III NSCLC.
Copyright ©ERS 2024.
Conflict of interest statement
Conflict of interest: S. Ramella reports payment for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from MSD Italia, Genetec, Amgen and Istituto Gentili, outside the submitted work; and has participated on a Data Safety Monitoring Board or Advisory Board for Roche and AstraZeneca, outside the submitted work. R.H. Petersen reports receiving speaker fees from Medtronic, AstraZeneca, AMBU and Medela, outside the submitted work; and participation on Advisory Boards for AstraZeneca, BMS, Roche and MSD, outside the submitted work. A. Bouterfas reports receiving payment for expert testimony from AstraZeneca, outside the submitted work. M.A. Heuvelmans reports being a member of IASLC Screening and Early Detection Committee (unpaid), and ERS Under-40 representative of the Thoracic Oncology Assembly (unpaid), outside the submitted work. W.H. van Geffen reports being a Board Member of Dutch Society of Respiratory Physicians (NVALT) Education Council Chair, and a member of ERS Long Range Planning Committee Thoracic Oncology; he is a local PI of clinical trials for MSD and Roche, outside the submitted work. The remaining authors have nothing to disclose.
Figures
References
-
- Global Cancer Observatory . Trachea, bronchus and lung fact sheet. https://gco.iarc.who.int/media/globocan/factsheets/cancers/15-trachea-br... Data last accessed: 1 February 2024.
Publication types
LinkOut - more resources
Full Text Sources