Diagnostic Accuracy of Cancer Antigen 15-3 as a Seromarker Among Recurrent Breast Carcinoma in Bangladesh
- PMID: 39360039
- PMCID: PMC11446177
- DOI: 10.7759/cureus.68448
Diagnostic Accuracy of Cancer Antigen 15-3 as a Seromarker Among Recurrent Breast Carcinoma in Bangladesh
Abstract
Background: The diagnosis of recurrent breast carcinoma is crucial for patient treatment. The present study aimed to assess the diagnostic accuracy of cancer antigen 15-3 (CA 15-3) as a sero-marker among recurrent breast carcinoma patients.
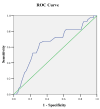
Methods: This prospective observational study evaluated the serum CA 15-3 among women (age ≥18 years) with recurrent breast carcinoma. The CA 15-3 was measured by the enzyme-linked immunosorbent assay (ELISA), and concentrations were stratified using a cut-off value of 30 U/mL. The receiver operating characteristic (ROC) curve observed that the sensitivity and specificity of the CA 15-3 cut-off value and the area under the AUROC curve demonstrate the goodness-of-fit of the prediction model.
Results: A total of 50 patients were recruited, with a mean age of 48.4 ±9.7years. The majority (n=28, 56.0%) of patients were 41 to 50 years old. Further, a total of 42 (84%) patients had high serum levels of CA 15-3, with a mean value of 72.7±9.5 U/mL. At the cut-off level of 30 U/mL, the ROC curve demonstrated sensitivity, specificity, positive predictive value, and negative predictive value of 95.7%, 69.4%, 84.1%, and 72.8%, respectively, to diagnose recurrent breast carcinoma. Nonetheless, the area under the ROC (AUROC) curve was 0.712, indicating a satisfactory fit for the prediction model.
Conclusion: We found that CA 15-3 level ≥30 U/mL is highly sensitive and specific as a seromarker for detecting recurrent breast cancer among the Bangladeshi population. We recommend routinely monitoring breast cancer survivors using CA 15-3 biomarkers.
Keywords: breast cancer research; ca 15-3; ca breast prognostic markers; high risk breast cancer; recurrence predictors.
Copyright © 2024, Kabir et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional review board of Dhaka Medical College and Hospital, Dhaka, Bangladesh issued approval ERC-DMC/ECC/2019/366. Ethical clearance was obtained from the institutional review board of Dhaka Medical College and Hospital, and informed consent was obtained from each study subject. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Receiver operating characteristic (ROC) curve analysis of the tumour markers CEA, CA 50 and CA 242 in pancreatic cancer; results from a prospective study.Br J Cancer. 1993 Apr;67(4):852-5. doi: 10.1038/bjc.1993.156. Br J Cancer. 1993. PMID: 8471445 Free PMC article.
-
Clinical utility of tumour marker velocity of cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in breast cancer surveillance.Breast. 2020 Aug;52:95-101. doi: 10.1016/j.breast.2020.05.005. Epub 2020 May 20. Breast. 2020. PMID: 32485607 Free PMC article.
-
Clinical value of serum tumour markers TPA, TPS, TAG 12, CA 15-3 and MCA in breast cancer diagnosis; results from a prospective study.Anticancer Res. 1994 Mar-Apr;14(2B):699-703. Anticancer Res. 1994. PMID: 8010729
-
The pretreatment plasma level and diagnostic utility of M-CSF in benign breast tumor and breast cancer patients.Clin Chim Acta. 2006 Sep;371(1-2):112-6. doi: 10.1016/j.cca.2006.02.033. Epub 2006 Apr 21. Clin Chim Acta. 2006. PMID: 16631152
-
Determination of the optimal cut-off value of serum prostate-specific antigen in the prediction of skeletal metastases on technetium-99m whole-body bone scan by receiver operating characteristic curve analysis.World J Nucl Med. 2020 Jul 1;19(3):255-259. doi: 10.4103/wjnm.WJNM_77_19. eCollection 2020 Jul-Sep. World J Nucl Med. 2020. PMID: 33354181 Free PMC article.
References
-
- The utility of serum tumor markers CEA and CA 15-3 for breast cancer prognosis and their association with clinicopathological parameters. Uygur MM, Gümüş M. Cancer Treat Res Commun. 2021;28:100402. - PubMed
-
- Sensitivity of CA 15-3, CEA and serum HER2 in the early detection of recurrence of breast cancer. Pedersen AC, Sørensen PD, Jacobsen EH, Madsen JS, Brandslund I. Clin Chem Lab Med. 2013;51:1511–1519. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials