Insight into the emerging insect to human pathogen Photorhabdus revealing geographic differences in immune cell tropism
- PMID: 39360318
- PMCID: PMC11444972
- DOI: 10.3389/fmicb.2024.1425909
Insight into the emerging insect to human pathogen Photorhabdus revealing geographic differences in immune cell tropism
Abstract
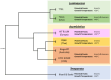
Background: Photorhabdus asymbiotica is a species of the insect pathogenic Photorhabdus genus that has been isolated as an etiological agent in human infections. Since then, multiple isolates have been identified worldwide; however, actual clinical infections have so far only been identified in North America, Australia, and Nepal. Previous research on the clinical isolates had shown that the strains differed in their behaviour when infecting cultured human cells.
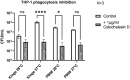
Methods: In this study, we investigate the differences between the pathogenic activities of P. asymbiotica isolates from different geographic locations. Pathogenicity was analysed using infection assays with both cultured cell lines (THP-1, CHO, and HEK cells) and primary immune cells, and peripheral blood mononuclear cells (PBMCs) isolated from human blood.
Results: Here, we present the findings from the Australian (Kingscliff) and North American (ATCC43949) clinical isolates, and non-clinical soilborne nematode isolates from Thailand (PB68) and Northern Europe (HIT and JUN) of P. asymbiotica. We also show the first findings from a new clinical isolate of P. luminescens (Texas), the first non-asymbiotica species to cause a human infection, confirming its ability to infect and survive inside human immune cells.
Conclusion: Here for the first time, we show how P. asymbiotica selectively infects certain immune cells while avoiding others and that infectivity varies depending on growth temperature. We also show that the tropism varies depending on the geographic location a strain is isolated from, with only the European HIT and JUN strains lack the ability to survive within mammalian cells in tissue culture.
Keywords: Photorhabdus; emerging pathogen; geographical localisation; human lymphocytes; tropism.
Copyright © 2024 Addison, Hapeshi, Wong, Connolly and Waterfield.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Boyles T. H., Wasserman S. (2015). Diagnosis of bacterial infection. S. Afr. Med. J. 105:419. doi: 10.7196/SAMJ.9647 - DOI
-
- Brugirard-Ricaud K., Duchaud E., Givaudan A., Girard P., Kunst F., Boemare N., et al. . (2005). Site-specific antiphagocytic function of the Photorhabdus luminescens type III secretion system during insect colonization. Cell. Microbiol. 7, 363–371. doi: 10.1111/j.1462-5822.2004.00466.x, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

