SPA Promotes Atherosclerosis Through Mediating Macrophage Foam Cell Formation-Brief Report
- PMID: 39360411
- PMCID: PMC11499019
- DOI: 10.1161/ATVBAHA.124.321460
SPA Promotes Atherosclerosis Through Mediating Macrophage Foam Cell Formation-Brief Report
Abstract
Background: Atherosclerosis is a progressive inflammatory disease in which macrophage foam cells play a central role in disease pathogenesis. SPA (surfactant protein A) is a lipid-associating protein involved with regulating macrophage function in various inflammatory diseases. However, the role of SPA in atherosclerosis and macrophage foam cell formation has not been investigated.
Methods: SPA expression was assessed in healthy and atherosclerotic human coronary arteries and the brachiocephalic arteries of wild-type or ApoE-deficient mice fed high-fat diets for 4 weeks. Hypercholesteremic wild-type and SPA-deficient mice fed a high-fat diet for 6 weeks were investigated for atherosclerotic lesions in vivo. In vitro experiments using RAW264.7 macrophages, primary resident peritoneal macrophages extracted from wild-type or SPA-deficient mice, and human monocyte-derived macrophages from the peripheral blood of healthy donors determined the functional effects of SPA in macrophage foam cell formation.
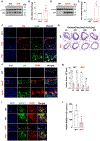
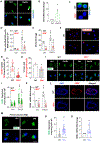
Results: SPA expression was increased in atherosclerotic lesions in humans and ApoE-deficient mice and in response to a proatherosclerotic stimulus in vitro. SPA deficiency reduced the lipid profiles induced by hypercholesterolemia, attenuated atherosclerosis, and reduced the number of lesion-associated macrophage foam cells. In vitro studies revealed that SPA deficiency reduced intracellular cholesterol accumulation and macrophage foam cell formation. Mechanistically, SPA deficiency dramatically downregulated the expression of scavenger receptor CD36 (cluster of differentiation antigen 36) cellular and lesional expression. Importantly, SPA also increased CD36 expression in human monocyte-derived macrophages.
Conclusions: Our results elucidate that SPA is a novel factor promoting atherosclerosis development. SPA enhances macrophage foam cell formation and atherosclerosis by increasing scavenger receptor CD36 expression, leading to increasing cellular OxLDL influx.
Keywords: atherosclerosis; cholesterol; lipoproteins, LDL; macrophages; receptors, scavenger.
Conflict of interest statement
None.
Figures
Update of
-
Surfactant protein A promotes atherosclerosis through mediating macrophage foam cell formation.bioRxiv [Preprint]. 2023 Mar 24:2023.03.23.533959. doi: 10.1101/2023.03.23.533959. bioRxiv. 2023. Update in: Arterioscler Thromb Vasc Biol. 2024 Nov;44(11):e277-e287. doi: 10.1161/ATVBAHA.124.321460. PMID: 36993244 Free PMC article. Updated. Preprint.
References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

