Phosphate promotes Arabidopsis root skewing and circumnutation through reorganisation of the microtubule cytoskeleton
- PMID: 39360424
- PMCID: PMC11579438
- DOI: 10.1111/nph.20152
Phosphate promotes Arabidopsis root skewing and circumnutation through reorganisation of the microtubule cytoskeleton
Abstract
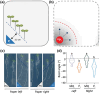
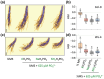
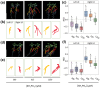
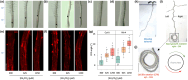
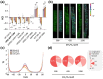
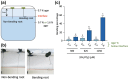
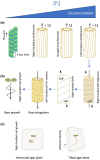
Phosphate (Pi) plays a key role in plant growth and development. Hence, plants display a range of adaptations to acquire it, including changes in root system architecture (RSA). Whether Pi triggers directional root growth is unknown. We investigated whether Arabidopsis roots sense Pi and grow towards it, that is whether they exhibit phosphotropism. While roots did exhibit a clear Pi-specific directional growth response, it was, however, always to the left, independent of the direction of the Pi gradient. We discovered that increasing concentrations of KH2PO4, trigger a dose-dependent skewing response, in both primary and lateral roots. This phenomenon is Pi-specific - other nutrients do not trigger this - and involves the reorganisation of the microtubule cytoskeleton in epidermal cells of the root elongation zone. Higher Pi levels promote left-handed cell file rotation that results in right-handed, clockwise, root growth and leftward skewing as a result of the helical movement of roots (circumnutation). Our results shed new light on the role of Pi in root growth, and may provide novel insights for crop breeding to optimise RSA and P-use efficiency.
Keywords: Arabidopsis; circumnutation; microtubule cytoskeleton; phosphate; root skewing.
© 2024 The Author(s). New Phytologist © 2024 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures



References
-
- Ahn PM, Keter JKA. 1986. Profile characteristics, and form and surface activity of inorganic phosphorus in a deep red Kenya coffee soil (Nitosol). European Journal of Soil Science 37: 89–91.
-
- Al‐Babili S, Bouwmeester HJ. 2015. Strigolactones, a novel carotenoid‐derived plant hormone. Annual Review of Plant Biology 66: 161–186. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

