Sex-specific post-inflammatory dysbiosis mediates chronic visceral pain in colitis
- PMID: 39360560
- PMCID: PMC11451282
- DOI: 10.1080/19490976.2024.2409207
Sex-specific post-inflammatory dysbiosis mediates chronic visceral pain in colitis
Abstract
Background: Despite achieving endoscopic remission, over 20% of inflammatory bowel disease (IBD) patients experience chronic abdominal pain. Visceral pain and the microbiome exhibit sex-dependent interactions, while visceral pain in IBD shows a sex bias. Our aim was to evaluate whether post-inflammatory microbial perturbations contribute to visceral hypersensitivity in a sex-dependent manner.
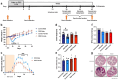
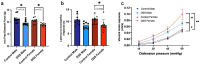
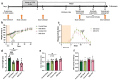
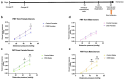
Methods: Males, cycling females, ovariectomized, and sham-operated females were given dextran sodium sulfate to induce colitis and allowed to recover. Germ-free recipients received sex-appropriate and cross-sex fecal microbial transplants (FMT) from post-inflammatory donor mice. Visceral sensitivity was assessed by recording visceromotor responses to colorectal distention. The composition of the microbiota was evaluated via 16S rRNA gene V4 amplicon sequencing, while the metabolome was assessed using targeted (short chain fatty acids - SCFA) and semi-targeted mass spectrometry.
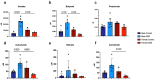
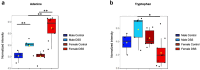
Results: Post-inflammatory cycling females developed visceral hyperalgesia when compared to males. This effect was reversed by ovariectomy. Both post-inflammatory males and females exhibited increased SCFA-producing species, but only males had elevated fecal SCFA content. FMT from post-inflammatory females transferred visceral hyperalgesia to both males and females, while FMT from post-inflammatory males could only transfer visceral hyperalgesia to males.
Conclusions: Female sex, hormonal status as well as the gut microbiota play a role in pain modulation. Our data highlight the importance of considering biological sex in the evaluation of visceral pain.
Keywords: DSS colitis; Microbiome; colorectal distention; fecal microbiota transplant; sex-differences; short-chain fatty acids; visceral pain.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
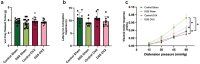


References
-
- Coward S, Benchimol EI, Kuenzig ME, Windsor JW, Bernstein CN, Bitton A, Jones JL, Lee K, Murthy SK, Targownik LE, et al. The 2023 impact of inflammatory bowel disease in Canada: epidemiology of IBD. J Can Assoc Gastroenterol. 2023;6(Supplement_2):S9–24. doi: 10.1093/jcag/gwad004. - DOI - PMC - PubMed
-
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939. - DOI - PMC - PubMed
-
- Fairbrass KM, Costantino SJ, Gracie DJ, Ford AC.. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(12):1053–1062. doi: 10.1016/S2468-1253(20)30300-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
