Darvadstrocel for complex perianal fistulas in Crohn's disease: A systematic review and meta-analysis
- PMID: 39360706
- PMCID: PMC11999041
- DOI: 10.1002/ueg2.12673
Darvadstrocel for complex perianal fistulas in Crohn's disease: A systematic review and meta-analysis
Abstract
Background: Local injection of darvadstrocel, a suspension of expanded adipose-derived allogenic mesenchymal stem cells, has been used for treatment-refractory perianal fistulas in Crohn's disease (CD).
Objective: This study aimed to investigate efficacy and safety of darvadstrocel for complex perianal fistulas in CD.
Methods: A systematic search was conducted through April 2024 in relevant databases for observational studies evaluating darvadstrocel. A random-effects meta-analysis model was used to calculate the pooled effect sizes (proportions or incidence rates [IRs]) with 95% confidence intervals (CIs) of effectiveness and safety outcomes. The risk of bias was evaluated using the Joanna Briggs Institute Critical Appraisal Tool. The I2 value assessed heterogeneity. Sensitivity and subgroup analyses were also conducted.
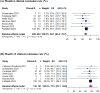
Results: Twelve studies were included with 595 patients. The pooled rate of patients achieving clinical remission, defined as fistula healing, was 68.1% at month 6 (95% CI 63.4-72.7) and 77.2% (95% CI 70.1-83.8) at month 12. Combined remission, defined as clinical remission and absence of collections >2 cm confirmed by magnetic resonance imaging, was reported in 60.6% and in 69.7% of patients at months 6 and 12, respectively. The rate of patients with treatment failure, defined as no clinical remission at the last follow-up (mean 18.7 months; SD 9.9), was 34.5%. Failure rate was independent of follow-up time (p = 0.85). For effectiveness outcomes, between-study heterogeneity was negligible. Subgroup analysis indicated that none of the covariates modified the treatment effect. Pooled IRs per 100 patient-years of adverse events (AE), serious AEs, perianal abscesses, and reoperations were 19.6, 3.2, 16.9 and 7.1, respectively.
Conclusion: Evidence from observational studies supports the efficacy and safety of darvadstrocel for complex perianal fistulas in CD. Studies have reported high fistula healing rates that can be sustained long-term in most patients, with negligible between-study heterogeneity, as well as a favorable safety profile.
Keywords: Crohn's disease; adipose‐derived allogenic mesenchymal stem cells; alofisel; darvadstrocel; inflammatory bowel disease; meta‐analysis; perianal fistulas.
© 2024 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
Carlos Taxonera has served as a speaker, consultant, and advisory board member for MSD, AbbVie, Pfizer, Takeda, Galapagos, Lilly, Fresenius Kabi, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, and Tillots. These activities were not related to the present work. The remaining authors have no conflicts of interest to declare.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

