Recent advances in the influenza virus vaccine landscape: a comprehensive overview of technologies and trials
- PMID: 39360831
- PMCID: PMC11629632
- DOI: 10.1128/cmr.00025-24
Recent advances in the influenza virus vaccine landscape: a comprehensive overview of technologies and trials
Abstract
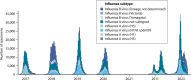
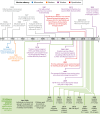
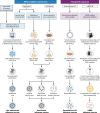
SUMMARYIn the United Kingdom (UK) in 2022/23, influenza virus infections returned to the levels recorded before the COVID-19 pandemic, exerting a substantial burden on an already stretched National Health Service (NHS) through increased primary and emergency care visits and subsequent hospitalizations. Population groups ≤4 years and ≥65 years of age, and those with underlying health conditions, are at the greatest risk of influenza-related hospitalization. Recent advances in influenza virus vaccine technologies may help to mitigate this burden. This review aims to summarize advances in the influenza virus vaccine landscape by describing the different technologies that are currently in use in the UK and more widely. The review also describes vaccine technologies that are under development, including mRNA, and universal influenza virus vaccines which aim to provide broader or increased protection. This is an exciting and important era for influenza virus vaccinations, and advances are critical to protect against a disease that still exerts a substantial burden across all populations and disproportionately impacts the most vulnerable, despite it being over 80 years since the first influenza virus vaccines were deployed.
Keywords: LAIV; adjuvanted; inactivated; influenza virus; live attenuated; prevention; recombinant; vaccine efficacy; vaccine safety; vaccine technologies.
Conflict of interest statement
T.W.C. has received speaker fees, honoraria, travel reimbursement, and equipment and consumables at discount or free of charge for the purposes independent of research, outside of this submitted study, from BioFire diagnostics, bioMérieux, QIAGEN, SenseBio Detection, and Inflammatix. He has received consultancy fees from BioFire, Cepheid, Synairgen Research, Roche, and Janssen. He has received honoraria for participation in advisory boards from Cepheid, Roche, Janssen, Shionogi, Sanofi, Seqirus, and GSK. He is a member of an independent data monitoring committee for a trial sponsored by Roche. He has acted as the UK chief investigator for a study sponsored by Janssen. He owns shares in Synairgen Research. J.S.N.-V.-T. was seconded to the Department of Health and Social Care, England (DHSC) between October 2017 and March 2022. After this assignment was completed, he reports lecture fees from AstraZeneca, Sanofi, Seqirus, and Gilead; also consulting fees from Roche, Seqirus, Janssen, Sanofi, and Moderna. The views expressed in this manuscript are those of its authors and not necessarily those of DHSC, its Agencies, or any of the above entities. J.S.T. has advised the Sanofi influenza vaccine program and received consultancy fees from Touchlight Genetics. H.L., T.P., and F.A. are employees of Sanofi and may hold stocks or shares and accrued pension rights in the company.
Figures
References
-
- World Health Organization . 2023. Influenza (Seasonal) factsheet. Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Retrieved 8 Aug 2024.
-
- Maleki F, Welch V, Lopez SMC, Cane A, Langer J, Enstone A, Markus K, Wright O, Hewitt N, Whittle I. 2023. Understanding the global burden of influenza in adults aged 18-64 years: a systematic literature review from 2012 to 2022. Adv Ther 40:4166–4188. doi: 10.1007/s12325-023-02610-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

