Intestinal Tuft Cells Are Enriched With Protocadherins
- PMID: 39360911
- PMCID: PMC11471013
- DOI: 10.1369/00221554241287267
Intestinal Tuft Cells Are Enriched With Protocadherins
Abstract
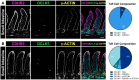
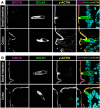
Intestinal tuft cells are rare cells that regulate diverse functions. They harbor chemosensory receptors and signal to the mucosal immune system in response to external stimuli, though their full function and structure remain unclear. Named for their apical "tuft" of long actin-rich microvilli, tuft cells facilitate chemoreception and other physiological responses. In enterocytes, microvilli are stabilized by intermicrovillar adhesion complexes (IMACs) composed of several proteins, including cadherin-related family member-2 (CDHR2) and cadherin-related family member-5 (CDHR5), Myosin 7b, and Usher syndrome type 1 C (USH1C). We hypothesized that IMACs would be enriched in tuft cells to regulate microvillar organization. Immunostaining of murine intestinal tissue revealed that CDHR2 and CDHR5 colocalize with the tuft cell markers, DCLK1, phospho-EGFR, advillin, and cytokeratin 18. CDHR2 was dispersed throughout murine tuft cells, while CDHR5 was concentrated on the apical surface. USH1C and Myosin 7b were present in tuft cells, but at lower levels. Human single-cell RNA sequencing revealed robust CDHR2 and CDHR5 expression in tuft cells in the small intestine and colon. Immunostaining of human intestinal tissue confirmed CDHR2 and CDHR5 localization to the apical surface of tuft cells. Our findings demonstrate that protocadherins are key components of murine and human intestinal tuft cells.
Keywords: IMAC; epithelium; gut; microvilli.
Conflict of interest statement
Competing InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Isomäki AM. A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta Pathol Microbiol Scand A. 1973; 240:1–35. - PubMed
-
- Luo XC, Chen ZH, Xue JB, Zhao DX, Lu C, Li YH, Li S-M, Du Y-W, Liu Q, Wang P, Liu M, Huang L. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc Natl Acad Sci U S A. 2019;116(12):5564–9. doi: 10.1073/pnas.1812901116. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

