Prevalence of Drug Resistance Associated Substitutions in Persons With Chronic Hepatitis C Infection and Virological Failure Following Initial or Re-treatment With Pan-genotypic Direct-Acting Antivirals: A Systematic Review and Meta-analysis
- PMID: 39361017
- PMCID: PMC11650865
- DOI: 10.1093/cid/ciae431
Prevalence of Drug Resistance Associated Substitutions in Persons With Chronic Hepatitis C Infection and Virological Failure Following Initial or Re-treatment With Pan-genotypic Direct-Acting Antivirals: A Systematic Review and Meta-analysis
Abstract
Background: The advent of short-course, curative treatment with direct-acting antivirals (DAA) has given promise for the global elimination of hepatitis C virus (HCV) infections by 2030. Virological failure occurs in 2%-12% of persons receiving curative DAA treatment and may be presaged by pre-existing polymorphisms or result from selection of drug resistant variants during therapy.
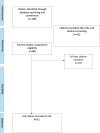
Methods: We conducted a systematic review to assess the prevalence of HCV resistance associated substitutions (RAS) among individuals with chronic hepatitis C infection who had virological failure following initial or re-treatment with pan-genotypic DAA regimens. We included 34 and 22 studies assessing RAS in people with virological failure published between January 2014 and July 2023. Pooled RAS prevalence was estimated using random-effects meta-analysis.
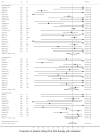
Results: The pooled prevalence of RAS in people with virological failure following initial DAA treatment was 78.0% (95% confidence interval [CI]: 62.0-92.0) for sofosbuvir/velpatasvir, 81.0% (95% CI: 67.0-93.0) for sofosbuvir/daclatasvir, and 79.0% (95% CI: 70.0-87.0) for glecaprevir/pibrentasvir, with a high prevalence of resistance to the NS5A inhibitors. Among those with virological failure following re-treatment regimens, RAS were present in 93.0% (95% CI: 83.0-99.0) for sofosbuvir/velpatasvir/voxilepravir and in 100% (95% CI: 92.0-100) for glecaprevir/pibrentasvir, with resistance driven by RAS to NS5A inhibitors.
Discussion: At least 1 RAS is present in a high proportion of the few individuals with virological failure following initial or re-treatment with pan-genotypic DAA regimens. There is a need for ongoing surveillance for DAA-associated resistance, to assess risk factors for their development and clinical impact to inform best practice strategies for re-treatment.
Keywords: chronic hepatitis C virus; initial treatment and retreatment; pan-genotypic direct acting agents; resistance-associated substitutions; treatment failure.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. W. I. reports personal fees from Roche diagnostics, Gilead Sciences, and Source Bioscience Limited outside of the submitted work and stock or stock options in GlaxoSmithKline. P. C. M. reports funding from Francis Crick Institute and University College London Biomedical Research Centre (BRC) and Wellcome Trust during the submitted work, as well as royalties from Oxford University Press and funding from GlaxoSmithKline outside of the submitted work. S. T. reports funding or personal fees ViiV Healthcare and Gilead Sciences outside of the submitted work. D. A. reports funding from Gilead Sciences and Pfizer outside of the submitted work. R. H. serves as the president of World Hepatitis Alliance. All other authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- World Health Organization (WHO) . Global hepatitis report 2024: action for access in low- and middle-income countries. Geneva, Switzerland: World Health Organization (WHO), 2024.
-
- World Health Organization (WHO) . Updated recommendations on treatment of adolescents and children with chronic HCV infection: policy brief. Geneva, Switzerland: World Health Organization (WHO), 2022. - PubMed
-
- World Health Organization (WHO) . Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva, Switzerland: World Health Organization (WHO), 2018. - PubMed
-
- Xie Z, Deng K, Xia Y, et al. Efficacy and safety of direct-acting antiviral therapies and baseline predictors for treatment outcomes in hepatitis C patients: a multicenter, real-world study in Guangdong, China. J Med Virol 2022; 94:4459–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

