Long-term safety and efficacy of ropeginterferon alfa-2b in Japanese patients with polycythemia vera
- PMID: 39361233
- PMCID: PMC11588802
- DOI: 10.1007/s12185-024-03846-5
Long-term safety and efficacy of ropeginterferon alfa-2b in Japanese patients with polycythemia vera
Erratum in
-
Correction: Long-term safety and efficacy of ropeginterferon alfa-2b in Japanese patients with polycythemia vera.Int J Hematol. 2025 Jul;122(1):159-161. doi: 10.1007/s12185-025-04002-3. Int J Hematol. 2025. PMID: 40377864 Free PMC article. No abstract available.
Abstract
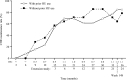
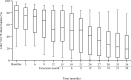
Ropeginterferon alfa-2b (ropegIFN), a new-generation interferon-based agent, has been approved in Japan for patients with polycythemia vera (PV) who are ineligible for or respond inadequately to conventional treatment. However, long-term outcomes with ropegIFN in Japanese patients have not been reported. This extension of a phase 2 study of ropegIFN in Japanese patients with PV aimed to determine its long-term safety/efficacy, and changes over time in JAK2 V617F allele burden. Here, we report data from the phase 2 study and subsequent extension over a period of 36 months. The primary endpoint was the complete hematologic response (CHR) maintenance rate without phlebotomy (hematocrit value < 45% without phlebotomy during the previous 12 weeks, platelet count ≤ 400 × 109/L, and white blood cell count ≤ 10 × 109/L). The CHR maintenance rates were 8/27 (29.6%), 18/27 (66.7%), and 22/27 (81.5%) at 12, 24, and 36 months, respectively. No thrombotic or hemorrhagic events occurred. The median allele burden change from baseline was - 74.8% at 36 months. All patients experienced adverse events; 25/27 (92.6%) experienced adverse drug reactions (ADRs), but no serious ADRs or deaths occurred. This interim analysis demonstrated the safety and efficacy of ropegIFN over 36 months in Japanese patients with PV.
Keywords: JAK2 V617F allele burden; Hematologic response; Molecular response; Polycythemia vera; Ropeginterferon alfa-2b.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: KK reports honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie G.K., Novartis Pharma K.K., PharmaEssentia Japan KK, Sanofi K.K., and Takeda Pharmaceutical Co., Ltd. YS reports research funding from Astellas Pharma Inc., Incyte Biosciences Japan G.K., Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co., Ltd., and Toyo Kohan Co., Ltd.; grants from Shojunkai Takeuchi Hospital; honoraria from Novartis Pharma K.K.; and participation on a data safety monitoring board or advisory board for Novartis Pharma K.K. AG reports research funding from Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., MSD K.K., Nihon Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; consulting fees from Alexion Pharmaceuticals, Inc., Chugai Pharmaceutical Co., Ltd., and PharmaEssentia Japan KK; honoraria from Alexion Pharmaceuticals, Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Kyowa Kirin Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Nihon Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Sanofi K.K., Sumitomo Pharma Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; and participation on a data safety monitoring board or advisory board for Alexion Pharmaceuticals, Inc., Chugai Pharmaceutical Co., Ltd., and PharmaEssentia Japan KK. KT reports research funding and consulting fees from Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., PharmaEssentia Japan KK, and Takeda Pharmaceutical Co., Ltd.; and honoraria from Alexion Pharmaceuticals, Inc., Kyowa Kirin Co., Ltd., MSD K.K., and Novartis Pharma K.K. M Ichii reports payments for presentations from Novartis Pharmaceuticals and payments for speakers bureaus from Sumitomo Pharma Co., Ltd. TI reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Asahi Kasei Pharma Co., Ltd., Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K., and PharmaEssentia Japan KK. SS reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie G.K., Amgen K.K., Asahi Kasei Pharma Co., Ltd., Novartis Pharma K.K., and Ono Pharmaceutical Co., Ltd. M Ito reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca K.K., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Incyte Biosciences Japan G.K., Janssen Pharmaceutical K.K., Novartis Pharma K.K., PharmaEssentia Japan KK, and Takeda Pharmaceutical Co., Ltd. OZ is an employee of PharmaEssentia Corporation USA. AQ is an employee of PharmaEssentia Corporation Taiwan. HK is an employee of PharmaEssentia Japan KK. TS is a board member of PharmaEssentia Japan KK. NK reports grants from Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Perseus Proteomics Inc., PharmaEssentia Japan KK, and Sumitomo Pharma Co., Ltd.; research funding from Meiji Seika Pharma Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; consulting fees from Japan Tobacco Inc., PharmaEssentia Japan KK, and Torii Pharmaceutical Co., Ltd.; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis Pharma K.K. and Takeda Pharmaceutical Co., Ltd.; and is a board member of PharmaEssentia Japan KK. KS reports research funding from AbbVie G.K., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Sumitomo Pharma Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; honoraria from Novartis Pharma K.K. and Takeda Pharmaceutical Co., Ltd.; and participation on a data safety monitoring board or advisory board for AbbVie G.K.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

