Ruxolitinib for steroid-refractory chronic graft-versus-host disease: Japanese subgroup analysis of REACH3 study
- PMID: 39361234
- PMCID: PMC11588829
- DOI: 10.1007/s12185-024-03850-9
Ruxolitinib for steroid-refractory chronic graft-versus-host disease: Japanese subgroup analysis of REACH3 study
Abstract
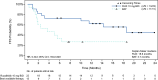
Ruxolitinib, a Janus kinase (JAK1-JAK2) inhibitor, has demonstrated safety and efficacy in patients with graft-versus-host disease (GvHD). This phase 3 randomized trial (REACH3) evaluated the efficacy and the safety of ruxolitinib 10 mg twice daily compared with investigator-selected best available therapy (BAT) in a subgroup of Japanese patients (n = 37) with steroid-refractory or dependent (SR/D) chronic GvHD. At data cut-off, treatment was ongoing in 17 patients and discontinued in 20. The overall response rate (complete or partial) at week 24 was greater with ruxolitinib than BAT (50% vs. 20%; odds ratio, 4.13 [95% CI, 0.90-18.9]). The best overall response rate (complete or partial response at any time point up to week 24) was higher with ruxolitinib than BAT (68.2% vs. 46.7%; odds ratio, 2.69 [95% CI, 0.66-10.9]). Ruxolitinib led to longer median failure-free survival than BAT (18.6 months vs. 3.7 months; hazard ratio, 0.34; [95% CI, 0.14-0.85]). The most common grade ≥ 3 adverse events up to week 24 were anemia (ruxolitinib: 22.7%; BAT: 6.7%) and pneumonia (22.7% and 20.0%, respectively). Ruxolitinib showed a higher response rate and improvement in failure-free survival in Japanese patients with SR/D chronic GvHD, with a safety profile consistent with the overall study population.
Keywords: Chronic graft-versus-host disease; JAK inhibitor; Japanese; REACH3; Ruxolitinib.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: SS, ND, MO, TK, TIshikawa and TIkeda have nothing to disclose. KF and MT received honoraria from Janssen. YO received honoraria from Novartis, Pfizer, Janssen, AsahiKasei, Abbvie, Astellas, Amgen, Chugai, Bristol-Myers Squibb, Symbio, MSD, Meiji Seika, Sumitomo Dainippon, Daiichi Sankyo, Nippon Shinyaku, IQVIA, KISSEI and Kyowa Kirin; research funding from Novartis, Pfizer, Janssen, Meiji Seika, Incyte, and Takara Bio. TG received honoraria from Novartis and Mallinckrodt. HN received honoraria from Novartis, Janssen, Chugai, Astellas, Bristol-Myers Squibb and Takeda; research funding from Novartis, Astellas, Bristol-Myers Squibb and Takeda. YM received research funding from from Asahi Kasei, Eisai, Otsuka, Kyowa Kirin, Taiho, Takeda, Chugai, Japan blood products, Nippon Kayaku, Nippon Shinyaku, Mallinckrodt, and Regimmune; and honoraria from Asahi Kasei, AstraZeneca, Astellas, Amgen, AbbVie, Viatris, Eisai, MSD, Otsuka, ONO, Gilead, Kyorin, Kyowa, Kissei, Konica, Sanofi, Celgene, Bristol-Myers Squibb, Bayer, CSL Behring, Daiichi Sankyo, Sumitomo Dainippon, Takeda, Terumo, Chugai, Nippon Shinyaku, Novartis, Pfizer, Mundipharma, Human Life CORD, Meiji Seika, Janssen and Yakult Honsha. KK received consulting fees from AbbVie, AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Janssen, and Novartis; honoraria from Bristol-Myers Squibb, Chugai, Sumitomo Pharma Co., Ltd., Janssen, Kyowa Kirin, MSD, Novartis and ONO; and research funding from AbbVie, MSD, Bristol-Myers Squibb, Janssen, Novartis, Chugai, Daiichi Sankyo, Eisai, Kyowa Kirin, and ONO. NU received honoraria from Novartis and Otsuka. AM, FS and TT are employees of Novartis Pharma K.K., Tokyo, Japan. TS is an employee of Novartis Pharma AG, Basel, Switzerland. TTeshima received honoraria from Novartis, Abbvie, Astellas, NIPPON SHINYAKU, Kyowa Kirin, Bristol-Myers Squibb, Sumitomo Pharma, Merck Sharp & Dohme, Celgene, Chugai, and Janssen; research funding from Astellas, Chugai, Fuji Pharma, Kyowa Kirin, Nippon Shinyaku, Asahi Kasei Pharma, Eisai, Sumitomo Pharma, ONO, Shionogi, Priothera SA, LUCA science and Otsuka; advisory board fees from Meiji Seika Pharma, Daiichi Sankyo, Asahi Kasei Pharma, Astellas, AstraZeneca, Takeda, Janssen, Toche Diagnostics, Sumitomo Pharma, Celgene, and Sanofi.
Figures


References
-
- Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am. 2011;25(1):101–16. - PubMed
-
- Berger M, Biasin E, Saglio F, Fagioli F. Innovative approaches to treat steroid-resistant or steroid refractory GVHD. Bone Marrow Transplant. 2008;42(Suppl 2):S101–5. - PubMed
-
- Ohwada C, Sakaida E, Igarashi A, Kobayashi T, Doki N, Mori T, et al. A Prospective Longitudinal Observation of the Incidence, Treatment, and Survival of Late Acute and Chronic Graft-versus-Host Disease by National Institutes of Health Criteria in a Japanese Cohort. Biol Blood Marrow Transplant. 2020;26(1):162–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

