Assessment of Antitachycardia Pacing in Primary Prevention Patients: The APPRAISE ATP Randomized Clinical Trial
- PMID: 39361311
- PMCID: PMC11581509
- DOI: 10.1001/jama.2024.16531
Assessment of Antitachycardia Pacing in Primary Prevention Patients: The APPRAISE ATP Randomized Clinical Trial
Erratum in
-
Error in Table.JAMA. 2025 Mar 11;333(10):911. doi: 10.1001/jama.2025.1887. JAMA. 2025. PMID: 39960740 Free PMC article. No abstract available.
Abstract
Importance: The emergence of novel programming guidelines that reduce premature and inappropriate therapies along with the availability of new implantable cardioverter-defibrillator (ICD) technologies lacking traditional endocardial antitachycardia pacing (ATP) capabilities requires the reevaluation of ATP as a first strategy in terminating fast ventricular tachycardias (VTs) in primary prevention ICD recipients.
Objective: To assess the role of ATP in terminating fast VTs in primary prevention ICD recipients with contemporary programming.
Design, setting, and participants: This global, prospective, double-blind, randomized clinical trial had an equivalence design with a relative margin of 35%. Superiority tests were performed at interim analyses and the final analysis if equivalence was not proven. Patients were enrolled between September 2016 and April 2021 at 134 sites in 8 countries, with the last date of follow-up on July 6, 2023. Patients were required to have an indication for a primary prevention ICD, including left ventricular ejection fraction less than or equal to 35%.
Interventions: Patients were randomized in a 1:1 ratio to receive ATP plus shock vs shock only.
Main outcomes and measures: The primary end point was time to first all-cause shock. Secondary end points included time to first appropriate shock, time to first inappropriate shock, all-cause mortality, and the composite of time to first all-cause shock plus all-cause mortality.
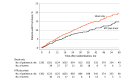
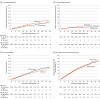
Results: A total of 2595 patients were randomized (mean age, 63.9 years; 22.4% were females). At a mean follow-up of 38 months, first all-cause shock occurred in 129 participants in the ATP plus shock group and 178 participants in the shock only group. The hazard ratio (HR) for the primary end point was 0.72 (95.9% CI, 0.57-0.92), with P = .005 for superiority of the ATP plus shock group over the shock only group. During follow-up in an intention-to-treat analysis, the total shock burden per 100 patient-years was not statistically different, at 12.3 and 14.9, respectively (P = .70).
Conclusions and relevance: The use of a single burst of ATP prior to shock in primary prevention ICD recipients with modern ICD detection programming prolonged the time to first all-cause ICD shock.
Trial registration: ClinicalTrials.gov Identifier: NCT02923726.
Conflict of interest statement
Figures


Comment in
-
Shock First or Pace First to Break Ventricular Tachycardia?: A New Layer of Complexity in ICD Shared Decision-Making.JAMA. 2024 Nov 26;332(20):1707-1708. doi: 10.1001/jama.2024.19416. JAMA. 2024. PMID: 39361313 No abstract available.
References
-
- Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883. doi: 10.1056/NEJMoa013474 - DOI - PubMed
-
- Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators . A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576-1583. doi: 10.1056/NEJM199711273372202 - DOI - PubMed
-
- Wathen MS, DeGroot PJ, Sweeney MO, et al. ; PainFREE Rx II Investigators . Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110(17):2591-2596. doi: 10.1161/01.CIR.0000145610.64014.E4 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

