Reactive microglia partially envelop viable neurons in prion diseases
- PMID: 39361421
- PMCID: PMC11601909
- DOI: 10.1172/JCI181169
Reactive microglia partially envelop viable neurons in prion diseases
Abstract
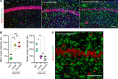
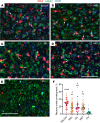
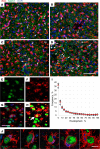
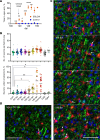
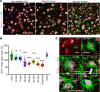
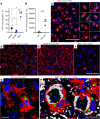
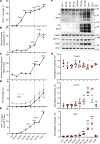
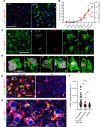
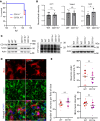
Microglia are recognized as the main cells in the central nervous system responsible for phagocytosis. The current study demonstrates that in prion disease, microglia effectively phagocytose prions or PrPSc during early preclinical stages. However, a critical shift occurred in microglial activity during the late preclinical stage, transitioning from PrPSc uptake to establishing extensive neuron-microglia body-to-body cell contacts. This change was followed by a rapid accumulation of PrPSc in the brain. Microglia that enveloped neurons exhibited hypertrophic, cathepsin D-positive lysosomal compartments. However, most neurons undergoing envelopment were only partially encircled by microglia. Despite up to 40% of cortical neurons being partially enveloped at clinical stages, only a small percentage of envelopment proceeded to full engulfment. Partially enveloped neurons lacked apoptotic markers, but showed signs of functional decline. Neuronal envelopment was independent of the CD11b pathway, previously associated with phagocytosis of newborn neurons during neurodevelopment. This phenomenon of partial envelopment was consistently observed across multiple prion-affected brain regions, various mouse-adapted strains, and different subtypes of sporadic Creutzfeldt-Jakob disease (sCJD) in humans. The current work describes a phenomenon of partial envelopment of neurons by reactive microglia in the context of an actual neurodegenerative disease, not a disease model.
Keywords: Infectious disease; Innate immunity; Neurodegeneration; Neuroscience; Prions.
Conflict of interest statement
Figures


Comment in
- Supportive care or exhausted neglect: the role of microglia at the end stage of prion disease
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

