Transcription factor KROX20 marks epithelial stem cell ancestors for hair follicle formation
- PMID: 39361422
- PMCID: PMC11601947
- DOI: 10.1172/JCI180160
Transcription factor KROX20 marks epithelial stem cell ancestors for hair follicle formation
Abstract
Epidermal stem cells control homeostasis and regeneration of skin and hair. In the hair follicle (HF) bulge of mammals, populations of slow-cycling stem cells regenerate the HF during cyclical rounds of anagen (growth), catagen (regression), and telogen (quiescence). Multipotent epidermal cells are also present in the HF above the bulge area, contributing to the formation and maintenance of sebaceous gland and upper and middle portions of the HF. Here, we report that the transcription factor KROX20 is enriched in an epidermal stem cell population located in the upper/middle HF. Expression analyses and lineage tracing using inducible Krox20-CreERT showed that Krox20-lineage cells migrate out of this HF region and contribute to the formation of the bulge in the HF, serving as ancestors of bulge stem cells. In vivo depletion of these cells arrests HF morphogenesis. This study identifies a marker for an epidermal stem cell population that is indispensable for hair homeostasis.
Keywords: Development; Mouse stem cells; Stem cells.
Figures
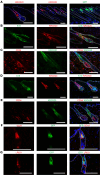

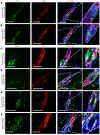




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

