Disruption of mitochondrial electron transport impairs urinary concentration via AMPK-dependent suppression of aquaporin 2
- PMID: 39361429
- PMCID: PMC11601893
- DOI: 10.1172/jci.insight.182087
Disruption of mitochondrial electron transport impairs urinary concentration via AMPK-dependent suppression of aquaporin 2
Abstract
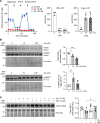
Urinary concentration is an energy-dependent process that minimizes body water loss by increasing aquaporin 2 (AQP2) expression in collecting duct (CD) principal cells. To investigate the role of mitochondrial (mt) ATP production in renal water clearance, we disrupted mt electron transport in CD cells by targeting ubiquinone (Q) binding protein QPC (UQCRQ), a subunit of mt complex III essential for oxidative phosphorylation. QPC-deficient mice produced less concentrated urine than controls, both at baseline and after type 2 vasopressin receptor stimulation with desmopressin. Impaired urinary concentration in QPC-deficient mice was associated with reduced total AQP2 protein levels in CD tubules, while AQP2 phosphorylation and membrane trafficking remained unaffected. In cultured inner medullary CD cells treated with mt complex III inhibitor antimycin A, the reduction in AQP2 abundance was associated with activation of 5' adenosine monophosphate-activated protein kinase (AMPK) and was reversed by treatment with AMPK inhibitor SBI-0206965. In summary, our studies demonstrated that the physiological regulation of AQP2 abundance in principal CD cells was dependent on mt electron transport. Furthermore, our data suggested that oxidative phosphorylation in CD cells was dispensable for maintaining water homeostasis under baseline conditions, but necessary for maximal stimulation of AQP2 expression and urinary concentration.
Keywords: Bioenergetics; Cell biology; Epithelial transport of ions and water; Mitochondria; Nephrology.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

