SEC31a-ATG9a Interaction Mediates the Recruitment of COPII Vesicles for Autophagosome Formation
- PMID: 39361436
- PMCID: PMC11600210
- DOI: 10.1002/advs.202405127
SEC31a-ATG9a Interaction Mediates the Recruitment of COPII Vesicles for Autophagosome Formation
Abstract
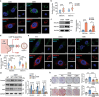
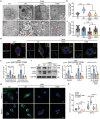
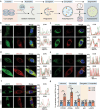
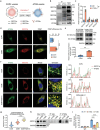
Autophagy plays an important role in determining stem-cell differentiation. During the osteogenic differentiation of mesenchymal stem cells (MSCs), autophagosome formation is upregulated but the reason is unknown. A long-standing quest in the autophagy field is to find the membrane origin of autophagosomes. In this study, cytoplasmic coat protein complex II (COPII) vesicles, endoplasmic reticulum-derived vesicles responsible for the transport of storage proteins to the Golgi, are demonstrated to be a critical source of osteoblastic autophagosomal membrane. A significant correlation between the number of COPII vesicle and the autophagy level is identified in the rat bone tissues. Disruption of COPII vesicles restrained osteogenesis and decreased the number and size of autophagosomes. SEC31a (an outer coat protein of COPII vesicle) is found to be vital to regulate COPII vesicle-dependent autophagosome formation via interacting with ATG9a of autophagosomal seed vesicles. The interference of Sec31a inhibited autophagosome formation and osteogenesis in vitro and in vivo. These results identified a novel mechanism of autophagosome formation in osteogenic differentiation of stem cells and identified SEC31a as a critical protein that mediates the interplay between COPII and ATG9a vesicles. These findings broaden the understanding of the regulatory mechanism in the osteogenic differentiation of MSCs.
Keywords: ATG9a; COPII vesicles; SEC31a; autophagosome formations; osteogenesis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nollet M., Santucci‐Darmanin S., Breuil V., Al‐Sahlanee R., Cros C., Topi M., Momier D., Samson M., Pagnotta S., Cailleteau L., Battaglia S., Farlay D., Dacquin R., Barois N., Jurdic P., Boivin G., Heymann D., Lafont F., Lu S., Dempster D. W., Carle G. F., Pierrefite‐Carle V., Autophagy 2014, 10, 1965. - PMC - PubMed
-
- Jaber F. A., Khan N. M., Ansari M. Y., Al‐Adlaan A. A., Hussein N. J., Safadi F. F., J. Cell. Physiol. 2019, 234, 12105. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
