Differences in COVID-19 Outpatient Antiviral Treatment Among Adults Aged ≥65 Years by Age Group - National Patient-Centered Clinical Research Network, United States, April 2022-September 2023
- PMID: 39361539
- PMCID: PMC11449269
- DOI: 10.15585/mmwr.mm7339a3
Differences in COVID-19 Outpatient Antiviral Treatment Among Adults Aged ≥65 Years by Age Group - National Patient-Centered Clinical Research Network, United States, April 2022-September 2023
Abstract
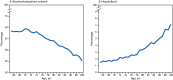
Adults aged ≥65 years experience the highest risk for COVID-19-related hospitalization and death, with risk increasing with increasing age; outpatient antiviral treatment reduces the risk for these severe outcomes. Despite the proven benefit of COVID-19 antiviral treatment, information on differences in use among older adults with COVID-19 by age group is limited. Nonhospitalized patients aged ≥65 years with COVID-19 during April 2022-September 2023 were identified from the National Patient-Centered Clinical Research Network. Differences in use of antiviral treatment among patients aged 65-74, 75-89, and ≥90 years were assessed. Multivariable logistic regression was used to estimate the association between age and nonreceipt of antiviral treatment. Among 393,390 persons aged ≥65 years, 45.9% received outpatient COVID-19 antivirals, including 48.4%, 43.5%, and 35.2% among those aged 65-75, 76-89, and ≥90 years, respectively. Patients aged 75-89 and ≥90 years had 1.17 (95% CI = 1.15-1.19) and 1.54 (95% CI = 1.49-1.61) times the adjusted odds of being untreated, respectively, compared with those aged 65-74 years. Among 12,543 patients with severe outcomes, 2,648 (21.1%) had received an outpatient COVID-19 antiviral medication, compared with 177,874 (46.7%) of 380,847 patients without severe outcomes. Antiviral use is underutilized among adults ≥65 years; the oldest adults are least likely to receive treatment. To prevent COVID-19-associated morbidity and mortality, increased use of COVID-19 antiviral medications among older adults is needed.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Faraz S. Ahmad reports receipt of consulting fees and editorial support from Pfizer. Thomas W. Carton reports receipt of grant support from the Task Force for Global Health. No other potential conflicts of interest were disclosed.
Figures
References
-
- CDC. COVID-19: underlying conditions and the higher risk for severe COVID-19. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. https://www.cdc.gov/covid/hcp/clinical-care/underlying-conditions.html#:...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

