Paternal age, de novo mutations, and offspring health? New directions for an ageing problem
- PMID: 39361588
- PMCID: PMC11630042
- DOI: 10.1093/humrep/deae230
Paternal age, de novo mutations, and offspring health? New directions for an ageing problem
Abstract
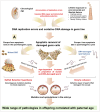
This Directions article examines the mechanisms by which a father's age impacts the health and wellbeing of his children. Such impacts are significant and include adverse birth outcomes, dominant genetic conditions, neuropsychiatric disorders, and a variety of congenital developmental defects. As well as age, a wide variety of environmental and lifestyle factors are also known to impact offspring health via changes mediated by the male germ line. This picture of a dynamic germ line responsive to a wide range of intrinsic and extrinsic factors contrasts with the results of trio studies indicating that the incidence of mutations in the male germ line is low and exhibits a linear, monotonic increase with paternal age (∼two new mutations per year). While the traditional explanation for this pattern of mutation has been the metronomic plod of replication errors, an alternative model pivots around the 'faulty male' hypothesis. According to this concept, the genetic integrity of the male germ line can be dynamically impacted by age and a variety of other factors, and it is the aberrant repair of such damage that drives mutagenesis. Fortunately, DNA proofreading during spermatogenesis is extremely effective and these mutant cells are either repaired or deleted by apoptosis/ferroptosis. There appear to be only two mechanisms by which mutant germ cells can escape this apoptotic fate: (i) if the germ cells acquire a mutation that by enhancing proliferation or suppressing apoptosis, permits their clonal expansion (selfish selection hypothesis) or (ii) if a genetically damaged spermatozoon manages to fertilize an oocyte, which then fixes the damage as a mutation (or epimutation) as a result of defective DNA repair (oocyte collusion hypothesis). Exploration of these proposed mechanisms should not only help us better understand the aetiology of paternal age effects but also inform potential avenues of remediation.
Keywords: antioxidant therapy; epigenetic mutation; genetic mutation; male ageing; oxidative stress; sperm DNA damage.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
R.J.A. is scientific advisor to Memphasys, a Sydney-based biotechnology company specializing in reproductive health.
Figures

References
-
- Aitken RJ. Role of sperm DNA damage in creating de-novo mutations in human offspring: the 'post-meiotic oocyte hypothesis. Reprod Biomed Online 2022a;45:109–124. - PubMed
-
- Aitken RJ. The Infertility Trap: Why Life Choices Impact Your Fertility and Why We Must Act Now. Cambridge, UK: Cambridge University Press, 2022c.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

