Calcineurin-mediated dephosphorylation stabilizes E2F1 protein by suppressing binding of the FBXW7 ubiquitin ligase subunit
- PMID: 39361641
- PMCID: PMC11474076
- DOI: 10.1073/pnas.2414618121
Calcineurin-mediated dephosphorylation stabilizes E2F1 protein by suppressing binding of the FBXW7 ubiquitin ligase subunit
Abstract
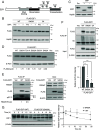
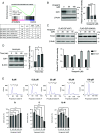
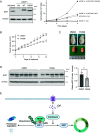
The transcription factor E2F1 serves as a regulator of the cell cycle and promotes cell proliferation. It is highly expressed in cancer tissues and contributes to their malignant transformation. Degradation by the ubiquitin-proteasome system may help to prevent such overexpression of E2F1 and thereby to suppress carcinogenesis. A detailed understanding of the mechanisms underlying E2F1 degradation may therefore inform the development of new cancer treatments. We here identified SCFFBXW7 as a ubiquitin ligase for E2F1 by comprehensive analysis. We found that phosphorylation of E2F1 at serine-403 promotes its binding to FBXW7 (F-box/WD repeat-containing protein 7) followed by its ubiquitination and degradation. Furthermore, calcineurin, a Ca2+/calmodulin-dependent serine-threonine phosphatase, was shown to stabilize E2F1 by mediating its dephosphorylation at serine-403 and thereby preventing FBXW7 binding. Treatment of cells with Ca2+ channel blockers resulted in downregulation of both E2F1 protein and the expression of E2F1 target genes, whereas treatment with the Ca2+ ionophore ionomycin induced upregulation of E2F1. Finally, the calcineurin inhibitor FK506 attenuated xenograft tumor growth in mice in association with downregulation of E2F1 in the tumor tissue. Impairment of the balance between the opposing actions of FBXW7 and calcineurin in the regulation of E2F1 abundance may therefore play an important role in carcinogenesis.
Keywords: E2F1; FBXW7; calcium; dephosphorylation; protein stability.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





References
-
- Gorgoulis V. G., et al. , Transcription factor E2F–1 acts as a growth-promoting factor and is associated with adverse prognosis in non-small cell lung carcinomas. J. Pathol. 198, 142–156 (2002). - PubMed
-
- Han S., et al. , E2F1 expression is related with the poor survival of lymph node-positive breast cancer patients treated with fluorouracil, doxorubicin and cyclophosphamide. Breast Cancer Res. Treat 82, 11–16 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

