MMTV RNA packaging requires an extended long-range interaction for productive Gag binding to packaging signals
- PMID: 39361708
- PMCID: PMC11449360
- DOI: 10.1371/journal.pbio.3002827
MMTV RNA packaging requires an extended long-range interaction for productive Gag binding to packaging signals
Abstract
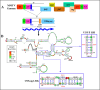
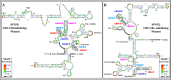
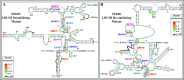
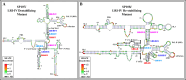
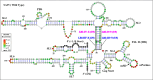
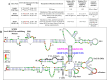
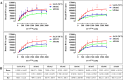
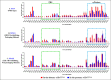
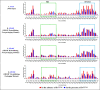
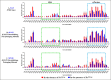
The packaging of genomic RNA (gRNA) into retroviral particles relies on the specific recognition by the Gag precursor of packaging signals (Psi), which maintain a complex secondary structure through long-range interactions (LRIs). However, it remains unclear whether the binding of Gag to Psi alone is enough to promote RNA packaging and what role LRIs play in this process. Using mouse mammary tumor virus (MMTV), we investigated the effects of mutations in 4 proposed LRIs on gRNA structure and function. Our findings revealed the presence of an unsuspected extended LRI, and hSHAPE revealed that maintaining a wild-type-like Psi structure is crucial for efficient packaging. Surprisingly, filter-binding assays demonstrated that most mutants, regardless of their packaging capability, exhibited significant binding to Pr77Gag, suggesting that Gag binding to Psi is insufficient for efficient packaging. Footprinting experiments indicated that efficient RNA packaging is promoted when Pr77Gag binds to 2 specific sites within Psi, whereas binding elsewhere in Psi does not lead to efficient packaging. Taken together, our results suggest that the 3D structure of the Psi/Pr77Gag complex regulates the assembly of viral particles around gRNA, enabling effective discrimination against other viral and cellular RNAs that may also bind Gag efficiently.
Copyright: © 2024 Prabhu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
A purine loop and the primer binding site are critical for the selective encapsidation of mouse mammary tumor virus genomic RNA by Pr77Gag.Nucleic Acids Res. 2021 May 7;49(8):4668-4688. doi: 10.1093/nar/gkab223. Nucleic Acids Res. 2021. PMID: 33836091 Free PMC article.
-
Biochemical and Functional Characterization of Mouse Mammary Tumor Virus Full-Length Pr77Gag Expressed in Prokaryotic and Eukaryotic Cells.Viruses. 2018 Jun 18;10(6):334. doi: 10.3390/v10060334. Viruses. 2018. PMID: 29912170 Free PMC article.
-
Structural basis of genomic RNA (gRNA) dimerization and packaging determinants of mouse mammary tumor virus (MMTV).Retrovirology. 2014 Nov 14;11:96. doi: 10.1186/s12977-014-0096-6. Retrovirology. 2014. PMID: 25394412 Free PMC article.
-
Retroviral Gag protein-RNA interactions: Implications for specific genomic RNA packaging and virion assembly.Semin Cell Dev Biol. 2019 Feb;86:129-139. doi: 10.1016/j.semcdb.2018.03.015. Epub 2018 Apr 1. Semin Cell Dev Biol. 2019. PMID: 29580971 Free PMC article. Review.
-
On the Selective Packaging of Genomic RNA by HIV-1.Viruses. 2016 Sep 12;8(9):246. doi: 10.3390/v8090246. Viruses. 2016. PMID: 27626441 Free PMC article. Review.
References
-
- Hunter E. Chapter 6, Viral Assembly. 6th Edition. Fields Virology. 6th Edition. 2013. p. 144–146.
-
- Flint SJ, Enquist LW, Racaniello VR, Skalka AM. Chapter 13. 2nd Edition. Principles of Virology. 2nd Edition. 2000. p. 466–474.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

