Characterization, ultrafiltration, depolymerization and gel formulation of ulvans extracted via a novel ultrasound-enzyme assisted method
- PMID: 39362034
- PMCID: PMC11483303
- DOI: 10.1016/j.ultsonch.2024.107072
Characterization, ultrafiltration, depolymerization and gel formulation of ulvans extracted via a novel ultrasound-enzyme assisted method
Abstract
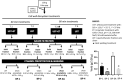
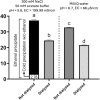
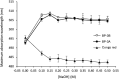
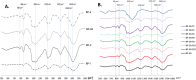
Sea lettuce, or Ulva spp., dominates global algal biomass and significantly contributes to "green tides.", representing a sustainable source for biomaterials. This study explores an innovative ultrasound-enzyme assisted extraction method with the novel Cellic® CTEC3 enzyme cocktail, applied for the first time in Ulva spp. succesfully enhancing ulvan release and extraction efficiency. Various processing methods, including ultrafiltration and dialysis, were employed to achieve higher ulvan purity. Dialyzation of ulvan resulted in a more purified product with a carbohydrate content up to 55.34 %, a sulfate content up to 21 %, and no glucose contamination. Liquid extracts were fractionated through ultrafiltration, with a 3 kDa MWCO yielding 93.51 % ulvan precipitate, representing 50.28 % of the total extractable ulvan. Sequential ultrafiltration concentrated ulvans but only partially modified their molecular weight distribution. Depolymerization using microwave and H2O2 shifted ulvans towards lower molecular weights, reducing high molecular weight residue. HPSEC confirmed pH-dependent aggregation behavior, with all isolated ulvans having molecular weights above 786 kDa. Hydrolysis methods were compared, with 2-hour 1 M TFA hydrolysis at 121 °C providing the best monosaccharide profile of ulvan. FTIR and NMR analyses showed preservation of sulfation. Rheology indicated biopolymeric behavior and stable gel formation. Ulvans demonstrated nutraceutical potential, being suitable for a low Na+ and high K+ diet, with a Na+:K+ ratio as low as 0.14, and were rich in Mg2+.
Keywords: Carbohydrates; Enzyme; Seaweed; Ultrasound; Ulva; Ulvan.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Sfriso A.A., Gallo M., Baldi F. Seasonal variation and yield of sulfated polysaccharides in seaweeds from the Venice Lagoon. Bot. Mar. 2017;60(3):339–349. doi: 10.1515/bot-2016-0063. - DOI
-
- Skriptsova A.V. Seasonal variations in the fucoidan content of brown algae from Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2016;42(4):351–356. doi: 10.1134/S1063074016040106. - DOI
-
- Shepherd V.A., Beilby M.J., Heslop D.J. Ecophysiology of the hypotonic response in the salt-tolerant charophyte alga Lamprothamnium papulosum. Plant, Cell Environ. 1999;22(4):333–346. doi: 10.1046/j.1365-3040.1999.00414.x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

