Adherence to Hygiene Protocols and Doxycycline Therapy in Ameliorating Lymphatic Filariasis Morbidity in an Endemic Area Post-Interruption of Disease Transmission in Ghana
- PMID: 39362213
- PMCID: PMC11448491
- DOI: 10.4269/ajtmh.24-0313
Adherence to Hygiene Protocols and Doxycycline Therapy in Ameliorating Lymphatic Filariasis Morbidity in an Endemic Area Post-Interruption of Disease Transmission in Ghana
Abstract
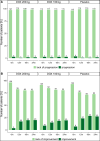
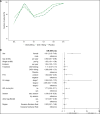
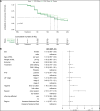
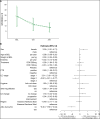
Filarial lymphedema (LE) remains a significant global problem despite the progress made toward elimination of lymphatic filariasis (LF). In Ghana, the main approach to LF is preventive chemotherapy, but this has minimal impact on individuals who have already developed LE. In 2018-2020, a 24-month randomized, double-blind, placebo-controlled trial was conducted to evaluate the efficacy of stringent hygiene measures using the Essential Package of Care with or without additional administration of doxycycline (DOX) to improve filarial leg LE. This study enrolled 356 participants with LE stages 1-3 from two districts in the Upper East Region of Ghana. In addition to regular training on appropriate care for their affected legs, participants were randomized to receive 6 weeks of either 200 mg/day DOX (n = 117), 100 mg/day DOX (n = 120), or matching placebo (n = 119). Participants were seen every 2 months, with clinical measurements done at 6, 12, 18, and 24 months to assess the status of affected legs. There was a trend toward later appearance of acute attacks after DOX, but surprisingly, DOX showed no effect on LE stage progression. In all groups, leg LE improvement was more common (DOX 200 mg: n = 23 [20%]; DOX 100 mg: n = 23 [19.5%]; placebo: n = 32 [27.4%]) than LE worsening (DOX 200 mg: n = 2 [1.7%]; DOX 100 mg: n = 3 [2.5%]; placebo: n = 2 [1.7%]). Overall, these data show a strong benefit from adherence to a strict hygiene protocol, with some added potential benefit for DOX in preventing acute attacks.
Conflict of interest statement
Disclosures: Ethical approval for the trial was obtained from the CHRPE of the School of Medical Sciences, KNUST, Kumasi; the GHS-ERC; Ghana Food and Drugs Authority (FDA); and the Ethics Committee of the Medical Faculty of the Rheinische Friedrich-Wilhelms-University Bonn. The ethical approval numbers/codes for the period of the trial were as follows: CHRPE/AP/525/17, CHRPE/AP/581/18, CHRPE/AP/632/19, CHRPE/AP/381/20, GHS-ERC:007/07/2017, FDA/CT/181, FDA/CT/181 and Bonn 359/171. Permission was also sought from community opinion leaders and elders prior to commencement of the trial. In addition, approvals were obtained from the Upper East Regional Health Directorate, the Kassena Nankana East Municipal, and the Kassena Nankana West District Health Directorates. Participants were educated and informed about the various procedures involved in screening, enrollment, and the overall purpose of the trial. Written consent was obtained from all adult participants either by thumbprinting or signing. Assents were completed for participants who were under 18 years of age. This trial was part of a larger project, “Tackling the Obstacle to Fight Filariasis and Podoconiosis” (TAKeOFF), sponsored by the German Ministry of Education and Research (BMBF;
Figures




Similar articles
-
LEDoxy-SL: A Placebo-Controlled, Double-Blind, Randomized, 24-Month Trial of Six Weeks of Daily Doxycycline Plus Hygiene-Based Essential Care for Reducing Progression of Filarial Lymphedema in Sri Lanka.Am J Trop Med Hyg. 2024 Jul 23;111(4_Suppl):52-65. doi: 10.4269/ajtmh.24-0050. Print 2024 Oct 1. Am J Trop Med Hyg. 2024. PMID: 39043165 Free PMC article. Clinical Trial.
-
Efficacy of Intensified Hygiene Measures with or without the Addition of Doxycycline in the Management of Filarial Lymphedema: A Randomized Double-Blind, Placebo-Controlled Clinical Trial in Tanzania.Am J Trop Med Hyg. 2024 Aug 27;111(4_Suppl):33-51. doi: 10.4269/ajtmh.24-0049. Print 2024 Oct 1. Am J Trop Med Hyg. 2024. PMID: 39191236 Free PMC article. Clinical Trial.
-
Effect of Adding a Six-Week Course of Doxycycline to Intensive Hygiene-Based Care for Improving Lymphedema in a Rural Setting of Mali: A Double-Blind, Randomized Controlled 24-Month Trial.Am J Trop Med Hyg. 2024 Jul 16;111(4_Suppl):22-32. doi: 10.4269/ajtmh.23-0908. Print 2024 Oct 1. Am J Trop Med Hyg. 2024. PMID: 39013374 Free PMC article. Clinical Trial.
-
Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD003753. doi: 10.1002/14651858.CD003753.pub4. Cochrane Database Syst Rev. 2019. PMID: 30620051 Free PMC article.
-
Filariasis: new drugs and new opportunities for lymphatic filariasis and onchocerciasis.Curr Opin Infect Dis. 2008 Dec;21(6):673-81. doi: 10.1097/QCO.0b013e328315cde7. Curr Opin Infect Dis. 2008. PMID: 18978537 Review.
Cited by
-
Time to consider doxycycline in the standard treatment of lymphatic filariasis? Emerging evidence on use of doxycycline as an adjunct to hygiene protocols.Trop Med Health. 2025 Mar 24;53(1):39. doi: 10.1186/s41182-025-00726-4. Trop Med Health. 2025. PMID: 40122880 Free PMC article.
-
Global Preventive and Management Strategies and Their Effectiveness in Patients With Secondary Lymphedema: A Scoping Review.Cureus. 2025 May 7;17(5):e83627. doi: 10.7759/cureus.83627. eCollection 2025 May. Cureus. 2025. PMID: 40486300 Free PMC article. Review.
-
Robust COVID-19 Vaccine Responses Despite Filarial Co-Infection: Insights from a Lymphatic Filariasis Cohort in Ghana.Vaccines (Basel). 2025 Mar 13;13(3):312. doi: 10.3390/vaccines13030312. Vaccines (Basel). 2025. PMID: 40266230 Free PMC article.
References
-
- World Health Organization , 2022. Lymphatic Filariasis. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis. Accessed February 10, 2023.
-
- World Health Organization , 2020. Control of Neglected Tropical Diseases. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/teams/control-of-neglected-tropical-diseases/lymphat.... Accessed February 10, 2023.
-
- Irvine MA, Stolk WA, Smith ME, Subramanian S, Singh BK, Weil GJ, Michael E, Hollingsworth TD, 2017. Effectiveness of a triple-drug regimen for global elimination of lymphatic filariasis: A modelling study. Lancet Infect Dis 17: 451–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

