Application of a deep-learning marker for morbidity and mortality prediction derived from retinal photographs: a cohort development and validation study
- PMID: 39362226
- PMCID: PMC11788570
- DOI: 10.1016/S2666-7568(24)00089-8
Application of a deep-learning marker for morbidity and mortality prediction derived from retinal photographs: a cohort development and validation study
Abstract
Background: Biological ageing markers are useful to risk stratify morbidity and mortality more precisely than chronological age. In this study, we aimed to develop a novel deep-learning-based biological ageing marker (referred to as RetiPhenoAge hereafter) using retinal images and PhenoAge, a composite biomarker of phenotypic age.
Methods: We used retinal photographs from the UK Biobank dataset to train a deep-learning algorithm to predict the composite score of PhenoAge. We used a deep convolutional neural network architecture with multiple layers to develop our deep-learning-based biological ageing marker, as RetiPhenoAge, with the aim of identifying patterns and features in the retina associated with variations of blood biomarkers related to renal, immune, liver functions, inflammation, and energy metabolism, and chronological age. We determined the performance of this biological ageing marker for the prediction of morbidity (cardiovascular disease and cancer events) and mortality (all-cause, cardiovascular disease, and cancer) in three independent cohorts (UK Biobank, the Singapore Epidemiology of Eye Diseases [SEED], and the Age-Related Eye Disease Study [AREDS] from the USA). We also compared the performance of RetiPhenoAge with two other known ageing biomarkers (hand grip strength and adjusted leukocyte telomere length) and one lifestyle factor (physical activity) for risk stratification of mortality and morbidity. We explored the underlying biology of RetiPhenoAge by assessing its associations with different systemic characteristics (eg, diabetes or hypertension) and blood metabolite levels. We also did a genome-wide association study to identify genetic variants associated with RetiPhenoAge, followed by expression quantitative trait loci mapping, a gene-based analysis, and a gene-set analysis. Cox proportional hazards models were used to estimate the hazard ratios (HRs) and corresponding 95% CIs for the associations between RetiPhenoAge and the different morbidity and mortality outcomes.
Findings: Retinal photographs for 34 061 UK Biobank participants were used to train the model, and data for 9429 participants from the SEED cohort and for 3986 participants from the AREDS cohort were included in the study. RetiPhenoAge was associated with all-cause mortality (HR 1·92 [95% CI 1·42-2·61]), cardiovascular disease mortality (1·97 [1·02-3·82]), cancer mortality (2·07 [1·29-3·33]), and cardiovascular disease events (1·70 [1·17-2·47]), independent of PhenoAge and other possible confounders. Similar findings were found in the two independent cohorts (HR 1·67 [1·21-2·31] for cardiovascular disease mortality in SEED and 2·07 [1·10-3·92] in AREDS). RetiPhenoAge had stronger associations with mortality and morbidity than did hand grip strength, telomere length, and physical activity. We identified two genetic variants that were significantly associated with RetiPhenoAge (single nucleotide polymorphisms rs3791224 and rs8001273), and were linked to expression quantitative trait locis in various tissues, including the heart, kidneys, and the brain.
Interpretation: Our new deep-learning-derived biological ageing marker is a robust predictor of mortality and morbidity outcomes and could be used as a novel non-invasive method to measure ageing.
Funding: Singapore National Medical Research Council and Agency for Science, Technology and Research, Singapore.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests THR and GL own stocks in MediWhale, to whom RetiAge was licensed. ZZ holds a National Health and Medical Research Council Investigator Grant (2010072) and owns two patents for biological age prediction from ocular images (AU2023903213A0 and CN114782361A). T-YW is a consultant for Aldropika Therapeutics, Bayer, Boehringer Ingelheim, Carl Zeiss, Genentech, Iveric Bio, Novartis, Oxurion, Plano, Roche, Sanofi, and Shanghai Henlius; is an inventor, holds patents, and is a co-founder of the start-up companies EyRiS and Visre; and has interests in, and develops digital solutions for, eye diseases, including diabetic retinopathy, outside of the submitted work. C-YC holds licences and receives consultation fees from MediWhale. All other authors declare no competing interests.
Figures
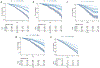


References
-
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017 - Highlights (ST/ESA/SER.A/397). 2017.
-
- Boyle JP, Honeycutt AA, Narayan KMV, Hoerger TJ, Geiss LS, Chen H, et al. Projection of Diabetes Burden Through 2050: Impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001. Nov 1;24(11):1936–40. - PubMed
-
- Gao X, Gào X, Zhang Y, Holleczek B, Schöttker B, Brenner H. Oxidative stress and epigenetic mortality risk score: associations with all-cause mortality among elderly people. Eur J Epidemiol. 2019. May;34(5):451–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

