Expression, purification, and characterization of diacylated Lipo-YcjN from Escherichia coli
- PMID: 39362470
- PMCID: PMC11543891
- DOI: 10.1016/j.jbc.2024.107853
Expression, purification, and characterization of diacylated Lipo-YcjN from Escherichia coli
Abstract
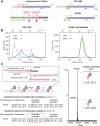
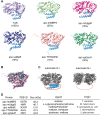
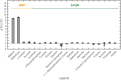
YcjN is a putative substrate binding protein expressed from a cluster of genes involved in carbohydrate import and metabolism in Escherichia coli. Here, we determine the crystal structure of YcjN to a resolution of 1.95 Å, revealing that its three-dimensional structure is similar to substrate binding proteins in subcluster D-I, which includes the well-characterized maltose binding protein. Furthermore, we found that recombinant overexpression of YcjN results in the formation of a lipidated form of YcjN that is posttranslationally diacylated at cysteine 21. Comparisons of size-exclusion chromatography profiles and dynamic light scattering measurements of lipidated and nonlipidated YcjN proteins suggest that lipidated YcjN aggregates in solution via its lipid moiety. Additionally, bioinformatic analysis indicates that YcjN-like proteins may exist in both Bacteria and Archaea, potentially in both lipidated and nonlipidated forms. Together, our results provide a better understanding of the aggregation properties of recombinantly expressed bacterial lipoproteins in solution and establish a foundation for future studies that aim to elucidate the role of these proteins in bacterial physiology.
Keywords: Escherichia coli; X-ray crystallography; bacterial lipoproteins; recombinant protein expression; substrate binding proteins.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Update of
-
Expression, purification, and characterization of diacylated Lipo-YcjN from Escherichia coli.bioRxiv [Preprint]. 2024 Sep 7:2024.09.05.611266. doi: 10.1101/2024.09.05.611266. bioRxiv. 2024. Update in: J Biol Chem. 2024 Nov;300(11):107853. doi: 10.1016/j.jbc.2024.107853. PMID: 39282304 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

