Pathogenic variants in CFAP46, CFAP54, CFAP74 and CFAP221 cause primary ciliary dyskinesia with a defective C1d projection of the central apparatus
- PMID: 39362668
- PMCID: PMC11651342
- DOI: 10.1183/13993003.00790-2024
Pathogenic variants in CFAP46, CFAP54, CFAP74 and CFAP221 cause primary ciliary dyskinesia with a defective C1d projection of the central apparatus
Abstract
Background: Primary ciliary dyskinesia is a rare genetic disorder caused by insufficient mucociliary clearance leading to chronic airway infections. The diagnostic guideline of the European Respiratory Society primarily recommends an evaluation of the clinical history (e.g. by the PICADAR prediction tool), nasal nitric oxide production rate measurements, high-speed videomicroscopy analysis of ciliary beating and an assessment of ciliary axonemes via transmission electron microscopy. Genetic testing can be implemented as a last step.
Aims: In this study, we aimed to characterise primary ciliary dyskinesia with a defective C1d projection of the ciliary central apparatus and we evaluated the applicability of the European Respiratory Society diagnostic guideline to this primary ciliary dyskinesia type.
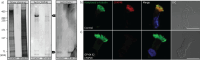
Methods: Using a high-throughput sequencing approach of genes encoding C1d components, we identified pathogenic variants in the novel primary ciliary dyskinesia genes CFAP46 and CFAP54, and the known primary ciliary dyskinesia gene CFAP221. To fully assess this primary ciliary dyskinesia type, we also analysed individuals with pathogenic variants in CFAP74.
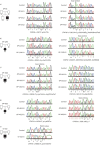
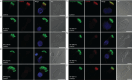
Results: Careful evaluation revealed that C1d-defective primary ciliary dyskinesia is associated with normal situs composition, normal nasal nitric oxide production rates, normal ciliary ultrastructure by transmission electron microscopy and normal ciliary beating by high-speed videomicroscopy analysis. Despite chronic respiratory disease, PICADAR does not reliably detect this primary ciliary dyskinesia type. However, we could show by in vitro ciliary transport assays that affected individuals exhibit insufficient ciliary clearance.
Conclusions: Overall, this study extends the spectrum of primary ciliary dyskinesia genes and highlights that individuals with C1d-defective primary ciliary dyskinesia elude diagnosis when using the current diagnostic algorithm. To enable diagnosis, genetic testing should be prioritised in future diagnostic guidelines.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures





Comment in
-
Genes take the lead: genetic testing becomes the gold standard for diagnosing primary ciliary dyskinesia.Eur Respir J. 2024 Dec 12;64(6):2401888. doi: 10.1183/13993003.01888-2024. Print 2024 Dec. Eur Respir J. 2024. PMID: 39667783 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
