Arabidopsis HAK5 under low K+ availability operates as PMF powered high-affinity K+ transporter
- PMID: 39362862
- PMCID: PMC11450230
- DOI: 10.1038/s41467-024-52963-6
Arabidopsis HAK5 under low K+ availability operates as PMF powered high-affinity K+ transporter
Abstract
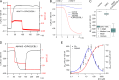
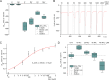
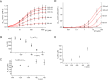
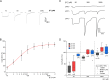
Plants can survive in soils of low micromolar potassium (K+) concentrations. Root K+ intake is accomplished by the K+ channel AKT1 and KUP/HAK/KT type high-affinity K+ transporters. Arabidopsis HAK5 mutants impaired in low K+ acquisition have been identified already more than two decades ago, the molecular mechanism, however, is still a matter of debate also because of lack of direct measurements of HAK5-mediated K+ currents. When we expressed AtHAK5 in Xenopus oocytes together with CBL1/CIPK23, no inward currents were elicited in sufficient K+ media. Under low K+ and inward-directed proton motive force (PMF), the inward K+ current increased indicating that HAK5 energetically couples the uphill transport of K+ to the downhill flux of H+. At extracellular K+ concentrations above 25 μM, the initial rise in current was followed by a concentration-graded inactivation. When we replaced Tyr450 in AtHAK5 to Ala the K+ affinity strongly decreased, indicating that AtHAK5 position Y450 holds a key for K+ sensing and transport. When the soil K+ concentration drops toward the range that thermodynamically cannot be covered by AKT1, the AtHAK5 K+/H+ symporter progressively takes over K+ nutrition. Therefore, optimizing K+ use efficiency of crops, HAK5 could be key for low K+ tolerant agriculture.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Clarkson, D. T. & Hanson, J. B. The mineral-nutrition of higher-plants. Annu. Rev. Plant Physiol. Plant Mol. Biol.31, 239–298 (1980). - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

