Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders
- PMID: 39362875
- PMCID: PMC11452214
- DOI: 10.1038/s41392-024-01952-8
Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders
Erratum in
-
Correction: Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders.Signal Transduct Target Ther. 2025 Dec 24;10(1):426. doi: 10.1038/s41392-025-02525-z. Signal Transduct Target Ther. 2025. PMID: 41444218 Free PMC article. No abstract available.
Abstract
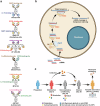
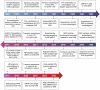
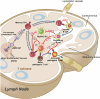
Autoimmune disorders are characterized by aberrant T cell and B cell reactivity to the body's own components, resulting in tissue destruction and organ dysfunction. Autoimmune diseases affect a wide range of people in many parts of the world and have become one of the major concerns in public health. In recent years, there have been substantial progress in our understanding of the epidemiology, risk factors, pathogenesis and mechanisms of autoimmune diseases. Current approved therapeutic interventions for autoimmune diseases are mainly non-specific immunomodulators and may cause broad immunosuppression that leads to serious adverse effects. To overcome the limitations of immunosuppressive drugs in treating autoimmune diseases, precise and target-specific strategies are urgently needed. To date, significant advances have been made in our understanding of the mechanisms of immune tolerance, offering a new avenue for developing antigen-specific immunotherapies for autoimmune diseases. These antigen-specific approaches have shown great potential in various preclinical animal models and recently been evaluated in clinical trials. This review describes the common epidemiology, clinical manifestation and mechanisms of autoimmune diseases, with a focus on typical autoimmune diseases including multiple sclerosis, type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, and sjögren's syndrome. We discuss the current therapeutics developed in this field, highlight the recent advances in the use of nanomaterials and mRNA vaccine techniques to induce antigen-specific immune tolerance.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Autoimmune Disease List, <https://autoimmune.org/disease-information/> (2024).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

