Pharmacodynamic, prognostic, and predictive biomarkers in severe and critical COVID-19 patients treated with sirukumab
- PMID: 39362933
- PMCID: PMC11452205
- DOI: 10.1038/s41598-024-74196-9
Pharmacodynamic, prognostic, and predictive biomarkers in severe and critical COVID-19 patients treated with sirukumab
Abstract
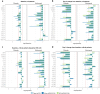
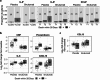
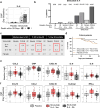
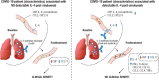
We examined candidate biomarkers for efficacy outcomes in hospitalized COVID-19 patients who were treated with sirukumab, an IL-6 neutralizing antibody, in a randomized, double-blind, placebo-controlled, phase 2 trial. Between May 2020 and March 2021, 209 patients were randomized (sirukumab, n = 139; placebo, n = 70); 112 had critical COVID-19. Serum biomarkers were evaluated for the pharmacodynamic effect of sirukumab and for their potential prognostic and predictive effect on time to sustained clinical improvement up to Day 28, clinical improvement at Day 28, and mortality at Day 28. The absence of detectable IL-4 increase and smaller increases in CCL13 post-baseline were most significantly associated with better response to sirukumab (versus placebo) treatment for all clinical efficacy outcomes tested, especially in patients with critical COVID-19. These data suggest that patients with critical COVID-19 without detectable sirukumab-induced IL-4 levels are more likely to benefit from sirukumab treatment. ClinicalTrials.gov Identifier: NCT04380961.
Keywords: Biomarkers; COVID-19; IL-4; IL-6; Inflammation.
© 2024. The Author(s).
Conflict of interest statement
K.C., M.C., L.V.W., E.V.L., and S.D.M. are employees of Janssen Pharmaceutica NV and may hold stock in Johnson & Johnson. K.T., J.A., and I.V. were employees of Janssen Pharmaceutical NV at the time the study was performed. M.J.L. and L.V. are employees of Janssen Research & Development, LLC and may hold stock in Johnson & Johnson. L.Z. is an employee of IQVIA.
Figures
References
-
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Drug for Treatment of COVID-19.) Accessed September 20. (2023). https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... (2021).
-
- ACTEMRA® (tocilizumab) injection, for intravenous or subcutaneous use [package insert] (Genentech, Inc., South San Francisco, CA, 2022).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

