Unlocking the C-centered ring-opening of phosphiranium ions for a straightforward entry to functionalized phosphines
- PMID: 39362940
- PMCID: PMC11449923
- DOI: 10.1038/s41467-024-53003-z
Unlocking the C-centered ring-opening of phosphiranium ions for a straightforward entry to functionalized phosphines
Abstract
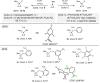
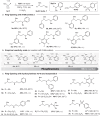
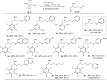
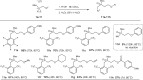
Phosphorus chemistry occupies a pivotal position in contemporary organic chemistry but significant synthetic challenges still endure. In this report, a class of electrophilic phosphiranium salts, bearing fluorinated benzyl quaternizing groups, is introduced for the direct synthesis of diversely β-functionalized phosphines. We show that, in comparison with regular quaternary phosphiranium salts, these species display the sought balance of excellent stability and high electrophilic reactivity that allow the unlocking of the C-centered ring-opening reactions with different classes of weak nitrogen-, sulfur- and oxygen protic nucleophiles.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Yudin, A. K. Aziridines and Epoxides in Organic Synthesis. (Wiley-VCH, Weinheim, 2006).
-
- Maji, B. Stereoselective Haliranium, Thiiranium and Seleniranium Ion-Triggered Friedel–Crafts-Type Alkylations for Polyene Cyclizations. Adv. Synth. Catal.361, 3453–3489 (2019). - DOI
-
- Vedejs, E. & Denmark, S. E. Lewis Base Catalysis in Organic Synthesis, 1153–1212. (Wiley-VCH, 2016). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

